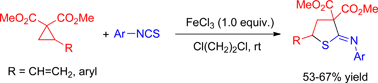Additions and corrections
FeCl3 promoted highly regioselective [3 + 2] cycloaddition of dimethyl 2-vinyl and aryl cyclopropane-1,1-dicarboxylates with aryl isothiocyanates
Huina Wang, Wei Yang, Hong Liu, Wei Wang and Hao Li
Org. Biomol. Chem., 2012, 10, 5032–5035 (DOI: 10.1039/C2OB25682G).
Amendment published 21st May 2013.
We published a manuscript entitled “FeCl3 promoted highly regioselective [3+2] cycloaddition of dimethyl 2-vinyl and aryl cyclopropane-1,1-dicarboxylates with aryl isothiocyanates” with ref. C2OB25682G in 2012. Upon further examination of the products formed in our reaction, we have found that the structures were misassigned. The correct product structures should be thioimidates instead of pyrrolidine-2-thiones. We wish to reference in this correction the Org. Lett. paper by Brian Stoltz (A. F. G. Goldberg, N. R. O’Connor, R. A. Craig and B. M. Stoltz, Org. Lett., 2012, 14, 5314) and acknowledge him for bringing this to our attention.
These are the schemes with the corrected structures:
Graphical Abstract:
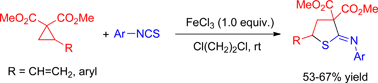
A FeCl3 promoted [3+2]-annulation of dimethyl 2-vinyl and arylcyclopropane-1,1-dicarboxylate with aryl isothiocyanates has been developed to give thioimidates regioselectively.
Table 1. Lewis acids screening for [3+2] cycloaddition reactions.
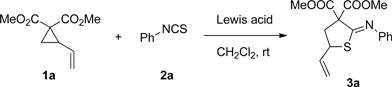
Table 2. Solvent effect for [3+2] cycloaddition reactions.
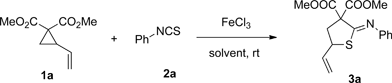
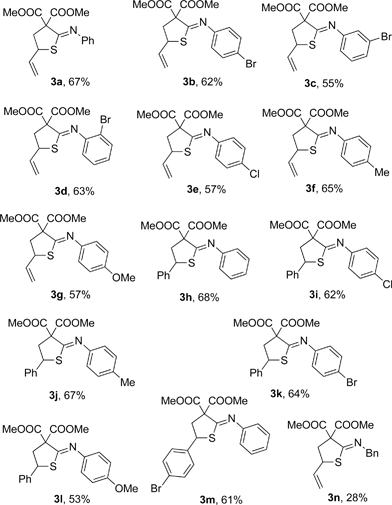
Figure 1. Scopes of Thioimidates.
Scheme 1. Proposed mechanism for the [3+2] cycloaddition reactions.

The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Back to article