A question I often get asked when dealing with the bleaching of coloured compounds is: why does the colour disappear?
One of my go-to demonstrations to address this question uses a chemical curiosity that came about following an 11-year dream of inventor Tim Kehoe to create a coloured bubble-blowing mixture. The resulting product (currently sold as Zubbles) works using leuco dyes. For a long time, producing coloured bubbles seemed almost impossible, since bubble walls are so thin dyes tend to sink to the base of the bubble.
Leuco dye molecules can exist in at least one coloured and one colourless form. The structures of these forms can be used to show students how organic dyes and pigments rely on delocalisation of electrons over extended molecular orbitals. This reduces the energy needed to promote electrons between energy levels to those corresponding to visible light frequencies. Any disruption to these extended delocalised systems can prevent a compound absorbing visible light, rendering it colourless.
Kit
Commercially produced leuco dye-based coloured bubble mix (Zubbles). Do not confuse Zubbles with other ‘washable’ coloured bubbles. These have attracted many complaints from users, after they found the product was anything but.
In front of the audience

This demonstration just requires you to blow the bubbles in front of your students. Encourage them to watch the bubbles land. When the bubbles pop on a surface, they will leave a coloured ‘stain’ that rapidly disappears.
If you have not had the opportunity to check an item of clothing for colour fastness, I would recommend blowing the bubbles up against a whiteboard. The dye decolorises very rapidly on the smooth, non-absorbent surface. You can even spread neat solution on the board and make it disappear with the rub of a finger.
If you’re feeling brave, you can blow bubbles up against a student wearing a white shirt or a lab coat. As the bubbles pop, it will look as if there is an ink stain on the item of clothing, but with a quick wipe, the stain will disappear. Always check the item of clothing beforehand as some weaves will absorb the dye rapidly away from the surface and the colour can persist longer (I’ve found that normal school polyester or polycotton shirts work better than the heavier weave of a lab coat, but of course the stakes are higher!). If a mark remains, a dab with a damp cloth should remove the colour. Any stains that do remain will disappear over time or in a normal wash cycle.
Teaching goal
Leuco dyes have been also been used in carbonless copy papers, ‘pink-to-white’ wall paints and erasable pens.
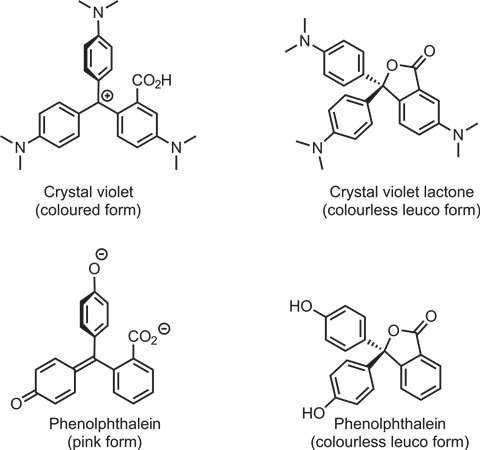
While leuco-dye based bubbles can be produced from numerous dyes in a variety of colours, the Zubbles product uses a dye similar in structure to crystal violet lactone. Functional modification of this compound allows for a variety of colours (although so far only two have made it to market). The simplicity of the crystal violet lactone molecule’s operation makes it particularly appealing to illustrate to students how organic dyes work.
Students will be familiar with the leuco and pink forms of phenolphthalein indicator; crystal violet dyes have similar structures, consisting of three benzene rings linked by a single carbon. Molecular modelling kits are particularly useful for showing students how the structures change. The structure of crystal violet is a little easier to interpret, having a planar resonance-stabilised carbocation that enables extended conjugation across all three rings (although steric clashing makes it unlikely that all three rings will be coplanar at once). There is a carboxyl group ortho to the bridging carbocation on one of the benzene rings. In the presence of oxygen, water or pressure, this closes a lactone (cyclic ester) to the central carbon to produce a tetrahedral centre and disrupt the conjugation. This also forces the benzene rings out of alignment.
It’s important to point out that the compound is, of course, still present but no longer visible.
The story behind the invention of these disappearing coloured bubbles is almost as entertaining and educational as the product itself: Tim Kehoe worked for many years to make the product a reality.



















No comments yet