An experiment that will illuminate the mysteries of ions! Create a cell of electrolyte and metals, and watch how the reactive and non-reactive metals form ion
This practical helps students to grasp the ideas around electromotive force found in chemicals.
This experiment should take 45 minutes.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Galvanometer or voltmeter (0–3 V)
- Wires x2
- Crocodile clips x2
Chemicals
- Sodium chloride solution
Access to strips or rods of various metals, including:
- Zinc
- Copper
- Iron
- Lead
- Magnesium
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection.
- Always wash hands after handling lead.
- Zinc is flammable and dangerous to aquatic life, see CLEAPSS Hazcard HC107.
- Lead is a reproductive toxin, see CLEAPSS Hazcard HC056.
- Magnesium is flammable, and reactive with water, see CLEAPSS Hazcard HC059a.
Procedure
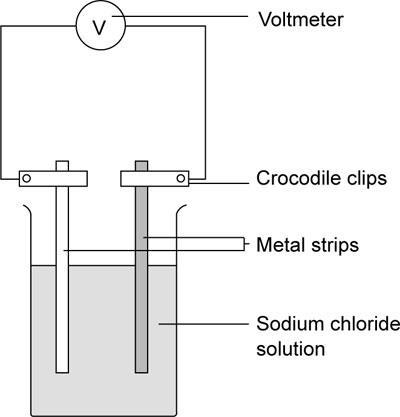
- Set up the apparatus as shown.
- Record the voltage.
- Try all the combinations of metals.
- Wash hands after handling lead.
- Complete table.
| Metals used | Which metal forms thepositive terminal (+ve) | Which metal forms thenegative terminal (-ve) | Voltage (V) |
|---|---|---|---|
| zinc and copper | |||
| copper and lead | |||
| lead and iron | |||
| zinc and lead | |||
| iron and magnesium | |||
| zinc and iron | |||
| zinc and magnesium | |||
| lead and magnesium | |||
| copper and magnesium | |||
| copper and iron |
Notes
Data logging sensors and software can be used in this experiment to provide a large screen display of the voltage changes. Connect a voltage sensor across the electrodes and get the software to show the reading using a meter or graph.
Metals high in the reactivity series have a tendency to release electrons to form ions.
Metals low in the series do not readily form ions, and their ions easily form metal atoms.
With zinc and copper:
Zn(s) → Zn2+(aq) + 2e– Cu2+(aq) + 2e– → Cu(s)
Questions
- Place zinc, magnesium, copper, lead, and iron in order of reactivity
Answers
- Magnesium, zinc, iron, lead, copper
Downloads
Electricity from chemicals - student sheet
PDF, Size 0.17 mbElectricity from chemicals - teacher notes
PDF, Size 0.12 mb
Additional information
This practical is part of our Classic chemistry experiments collection.


















1 Reader's comment