Try this class practical to illustrate the idea of competition reactions between metals and carbon
In this experiment, students heat two metal oxides with powdered charcoal. If the carbon is more reactive than the metal it will remove the oxygen from the metal oxide and leave traces of the metal in the reaction vessel. Students try lead(II) oxide first, before using a slightly modified technique to try copper(II) oxide.
The solids may be dispensed in plastic weighing dishes. It is best not to issue all three solids at the same time because students may confuse the charcoal and the copper oxide.
The experiment should take about 30 minutes.
Equipment
Apparatus
- Eye protection
- Small, hard glass test tube (ignition tubes), x3 (see note 6 below)
- Test tube holder
- Test tube rack
- Spatula
- Plastic weighing dish (boat)
- Heat resistant mat
Chemicals
- Powdered charcoal, about 2 g
- Copper(II) oxide (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), about 1 g
- Lead(II) oxide (TOXIC, DANGEROUS FOR THE ENVIRONMENT), about 1 g
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. The room should be well-ventilated.
- Powdered charcoal, C(s) – see CLEAPSS Hazcard HC021.
- Copper(II) oxide, CuO(s), (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026.
- Lead(II) oxide, PbO(s), (TOXIC, DANGEROUSFOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC056.
- Test tubes made of heat resistant borosilicate glass (Pyrex or similar) must be used. Test tubes with a capacity of about 10 cm3 are ideal. It is important that the test tubes are dry. Heating lead and its compounds strongly in glass often results in the lead compounds fusing into the glass, rendering the test tube impossible to reuse. If this is an issue, old but unstained test tubes can be used and discarded after this use.
Procedure
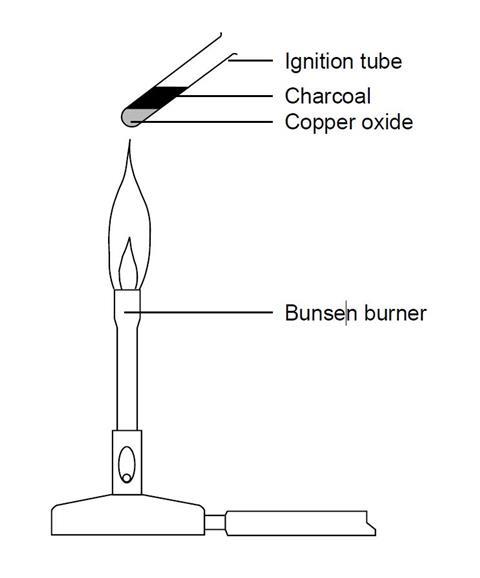
Experiment 1: lead(II) oxide
- Transfer one small spatula measure of lead(II) oxide to the empty weighing dish.
- Add one spatula measure of charcoal powder.
- Mix the two powders together using a spatula.
- Transfer the mixture into a hard-glass test tube and strongly heat this mixture for five minutes in a Bunsen flame.
- Allow the test tube to cool in its holder on a heat resistant mat.
- Tip the cooled mixture out onto the heat resistant mat.
Experiment 2: copper(II) oxide
- Transfer one spatula measure of copper(II) oxide to a hard glass test tube.
- Carefully add one spatula of charcoal powder on top of the copper oxide without any mixing.
- Strongly heat these two layers for five minutes in a Bunsen flame.
- Allow to cool and then look closely at where the powders meet in the test tube.
Teaching notes
In each case the students should look for signs that reaction has occurred producing the metal. The copper should be obvious from its colour. The lead may be less obvious; it may appear as globules or as grey powder.
The reactions confirm the place of carbon in the reactivity series, above lead and copper, as it reduces the metal oxides to the metals and is itself oxidised to carbon dioxide:
CuO(s) + C(s) → Cu(s) + CO2(g) and PbO(s) + C(s) → Pb(s) + CO2(g).
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet