Find out what happens when electricity passes through molten compounds… with water present
When electricity passes through molten compounds, like sodium chloride, the ions move towards the electrode of opposite charge.
This experiment should take 60 minutes.
Equipment
Apparatus
- Eye protection
- Beaker, 100 cm3
- Electrodes (S-shaped are preferable)
- Small test tubes for gas collection
- Two leads
- Two crocodile clips
- DC power supply (6 V is reasonable)
Chemicals
- Universal indicator paper
Access to solutions of :
- Sodium chloride 0.5 mol dm–3
- Copper chloride 0.5 mol dm–3
- Potassium iodide 0.5 mol dm–3
- Sodium bromide 0.5 mol dm–3
- Potassium sulfate 0.5 mol dm–3
- Copper(II) sulfate 0.5 mol dm–3
- Silver nitrate 0.1 mol dm–3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Always wear eye protection
- Chlorine is toxic and harmful to the lungs, eyes and respiratory tract. See CLEAPSS Hazcard HC022a.
- Bromine vapour is an irritant and very toxic if inhaled. See CLEAPSS Hazcard HC015b.
- Iodine is harmful by skin contact. See CLEAPSS Hazcard HC045.
- Hydrogen is extremely flammable. See CLEAPSS Hazcard HC048.
- Oxygen supports combustion. See CLEAPSS Hazcard HC069.
- Ensure good ventilation.
- Do not allow chlorine or bromine vapour to be produced for very long.
Procedure
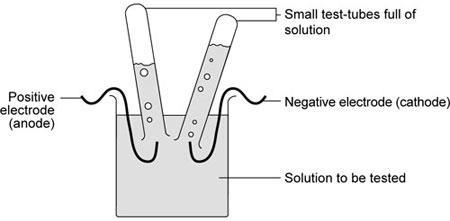
- Set up the apparatus as shown.
- Switch on and observe what happens.
- Try to identify the gases produced (if any).
Notes
Teachers may wish to show students how to fill and invert the small test-tubes over the electrodes.
With care, these can be inverted without spilling the liquid. Using small tubes filled with water rather than the test solution is safer.
These are then inverted into the solution to be tested.
The tubes may be clamped or supported by rubber bands wound round the test-tubes and round the electrode.
The class results can be pooled as there will not be time to test all the solutions individually.
Students may need reminding of, or introducing to, the common tests for hydrogen, oxygen, chlorine, bromine and iodine.
Questions
- What type of element is formed at the negative electrode?
- What type of element is formed at the positive electrode?
- Your table of results should show some products, which could not come from the compound itself that was electrolysed. Where could these other products have come from?
- Write a general rule for the products formed at (a) the cathode and (b) the anode.
Answers
- Metals or hydrogen.
- Non-metals.
- From the water.
- (a) The metal is produced if it is lower than hydrogen in the reactivity series, otherwise hydrogen is produced. (b) Halides give halogens, sulfates and nitrates give oxygen.
Downloads
The electrolysis of solutions – student sheet
PDF, Size 0.14 mbThe electrolysis of solutions – teacher notes
PDF, Size 0.13 mb
Additional information
This practical is part of our Classic chemistry experiments collection.


















No comments yet