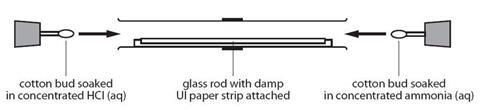
Place concentrated ammonia solution on a pad in one end of a tube and concentrated hydrochloric acid on a pad at the other and watch as the two gases diffuse far enough to meet and form a ring of solid ammonium chloride
This demonstration is best performed in a fume cupboard. A black background, such as a sheet of black sugar paper, behind the demonstration helps the white ring to be seen more clearly. Actually performing the demonstration takes only a few minutes.
Equipment
Apparatus
- Eye protection (goggles)
- Access to a fume cupboard
- Protective gloves (preferably nitrile)
- A length of glass tube about half a metre long with an inside diameter of about 2 cm (note 1)
- Retort stands with bosses and clamps x2
- Small wads of cotton wool x2
- Bungs x2 (to fit into the ends of the glass tube)
- Strip of universal indicator paper (optional)
Chemicals
- Concentrated hydrochloric acid (CORROSIVE), a few cm3 (note 2)
- 880 ammonia solution (CORROSIVE, DANGEROUS FOR THE ENVIRONMENT), a few cm3 (note 2)
- Acetone (FLAMMABLE), a few cm3 (optional, note 1)
Equipment notes
- It is very important that the tube is clean and completely dry for this experiment. If necessary, the tube can be dried by pushing a cotton wool pad soaked in acteone through the tube and leaving it for a few minutes.
- The concentrated hydrochloric acid and the 880 ammonia solution are easier to handle in small bottles than in Winchesters (large bottles) for this demonstration.
Health, safety and technical notes
- Read our standard health and safety guidance
- The demonstrator should wear goggles and protective gloves.
- Concentrated hydrochloric acid, HCl(aq), (CORROSIVE) – see CLEAPSS Hazcard HC047a. Produces hydrogen chloride gas, HCl(g), (TOXIC, CORROSIVE) – see CLEAPSS Hazcard HC049.
- 880 ammonia solution, NH3(aq), (CORROSIVE, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC006. Produces ammonia gas, NH3(g), (TOXIC) – see CLEAPSS Hazcard HC005. Care should be taken when opening the bottle of ammonia solution, particularly on hot days when pressure can build up in the bottle. If the bottle of ammonia is kept for a long time, its concentration may decrease which will lessen the effectiveness of the demonstration.
Procedure
- Working in the fume cupboard, clamp the glass tube at either end, ensuring that it is horizontal.
- Open the bottle of ammonia solution cautiously, pointing the bottle away from both you and the audience. Open the bottle of hydrochloric acid and hold the stopper near the mouth of the ammonia bottle. Note the white clouds of ammonium chloride that form.
- Put one of the cotton wool wads in the mouth of the ammonia bottle and carefully invert it to soak one side of it. Push the soaked end into one end of the glass tube. Replace the lid on the bottle.
- Repeat this procedure quickly with a second wad of cotton wool and hydrochloric acid. Put the cotton wool wad into the other end of the glass tube.
- Putting bungs into the ends of the glass tube will reduce the quantity of the gases which escape and therefore the smell. Once assembled, the tube can be removed from the fume cupboard.
- Watch the tube and observe a ring of white powder forming near the middle of the tube. This is ammonium chloride.
Teaching notes
The reaction which is taking place is:
ammonia + hydrogen chloride → ammonium chloride
NH3 (g) + HCl (g) → NH4Cl (s)
It typically takes just a few minutes for the ring to form, but the exact time will depend on the dimensions of the tube, the amount of the solutions which are put on the cotton wool wads and the temperature of the room.
The ring usually forms nearer to the hydrochloric acid end of the tube because hydrogen chloride diffuses more slowly than ammonia. This is because hydrogen chloride has almost twice the molecular weight of ammonia, and the rate of diffusion is inversely proportional to the square root of the molecular mass of the gas.
It is worth noting that the rate of diffusion is not the same as the speed at which the gas molecules travel (which is hundreds of meters per second). The gas molecules follow a zig-zag path through the tube as they collide with the air molecules in the tube.
The purpose of the glass tube is to eliminate air currents and to see if the gas molecules will move on their own.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet