Demonstrate that diffusion takes place in liquids by allowing lead nitrate and potassium iodide to form lead iodide as they diffuse towards each other in this practical
In this experiment, students place colourless crystals of lead nitrate and potassium iodide at opposite sides of a Petri dish of deionised water. As these substances dissolve and diffuse towards each other, students can observe clouds of yellow lead iodide forming, demonstrating that diffusion has taken place.
This practical activity takes around 30 minutes.
Equipment
Apparatus
- Eye protection
- Petri dish
- Forceps
- White tile or piece of white paper
Chemicals
- Lead nitrate (TOXIC, DANGEROUS FOR THE ENVIRONMENT), 1 crystal
- Potassium iodide, 1 crystal
- Deionised water
Greener alternatives
To reduce the use of toxic chemicals in this experiment you can conduct the experiment in microscale, using drops of water on a laminated sheet, find full instructions and video here, and/or use a less toxic salt than lead nitrate, eg sodium carbonate and barium chloride. More information is available from CLEAPSS.
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Lead nitrate, Pb(NO3)2(s), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC057a.
- Potassium iodide, KI(s) – see CLEAPSS Hazcard HC047b.
Procedure
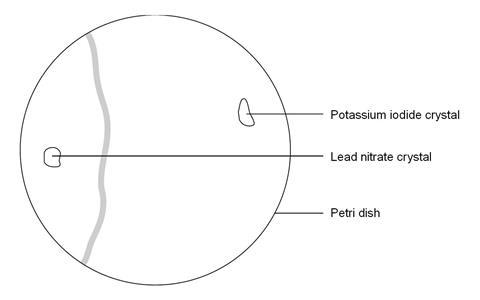
- Place a Petri dish on a white tile or piece of white paper. Fill it nearly to the top with deionised water.
- Using forceps, place a crystal of lead nitrate at one side of the petri dish and a crystal of potassium iodide at the other.
- Observe as the crystals begin to dissolve and a new compound is formed between them.
Teaching notes
The lead nitrate and potassium iodide each dissolve and begin to diffuse through the water. When the lead ions and iodide ions meet they react to form solid yellow lead iodide which precipitates out of solution.
lead nitrate + potassium iodide → lead iodide + potassium nitrate
Pb(aq) + 2I–(aq) → PbI2(s)
The precipitate does not form exactly between the two crystals. This is because the lead ion is heavier and diffuses more slowly through the liquid than the iodide ion.
Another experiment – a teacher demonstration providing an example of a solid–solid reaction – involves the same reaction but in the solid state.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















1 Reader's comment