Use this colourful practical to introduce students to the electrolysis of brine, or sodium chloride solution
In this experiment, students observe what happens during the electrolysis of brine (sodium chloride solution), using universal indicator to help them follow the reaction that takes place.
The experiment works well if students are directed to make detailed observations and then attempt to explain for themselves what they think is happening.
The main issue is likely to be the availability of sufficient U-shaped test tubes.
Equipment
Apparatus
- Eye protection
- U-shaped test tube
- Clamp and clamp stand
- Carbon electrodes and electrode holders, x2 (see note 8 below)
- Electrical leads, x2
- Power pack (low voltage, DC)
- Beaker, 100 cm3
- Spatula
- Stirring rod
Chemicals
- Sodium chloride (table salt) (see note 6 below)
- Universal indicator solution (FLAMMABLE)
- Distilled water (see note 7)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Hydrogen, H2 (g), (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC048.
- Chlorine, Cl2 (g), (TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC022a.
- Sodium hydroxide, NaOH(aq), (CORROSIVE) – see CLEAPSS Hazcard HC091a.
- The products of the electrolysis of the salt solution are all more hazardous than the starting materials. Hydrogen is EXTREMELY FLAMMABLE, chlorine is TOXIC and DANGEROUS FOR THE ENVIRONMENT, and sodium hydroxide is CORROSIVE. Ensure that the current is turned off a soon as a trace of chlorine is detected. Chlorine (TOXIC, DANGEROUS FOR THE ENVIRONMENT) can be a problem for asthmatic pupils. If the directions in the procedure notes are followed then very little chlorine is produced. Sodium hydroxide is CORROSIVE. Ensure that students wear eye protection, especially when they are clearing up the experiment.
- If distilled water is a problem, then tap water could be used. But it may affect the colours produced, especially in areas with hard water.
- If electrode holders are not available, another suitable means of securing the electrodes could be used. Do not use bungs because the products are gases.
Procedure
- Put about 75 cm3 distilled water into the beaker. Add about two heaped spatulas of sodium chloride.
- Stir until the salt dissolves. Then add several drops of universal indicator solution. Stir to mix thoroughly. You need enough indicator to give the water a reasonable depth of green colour.
- Pour coloured salt solution into the U-shaped test tube and clamp it as shown in the diagram.
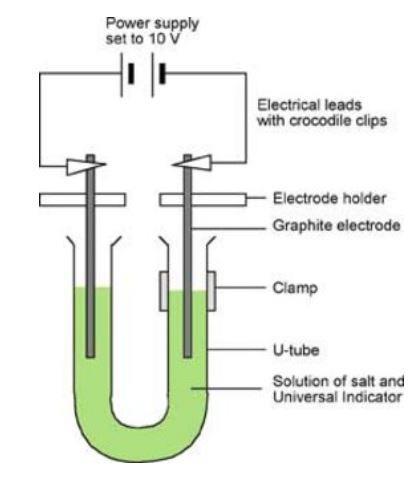
- Wash the carbon electrodes carefully in distilled water and then fix them so that there is about 3 cm of electrode in each side of the U-tube – see diagram. This is most easily done using electrode holders.
- Attach leads and connect to a power pack set to 10 V (DC).
- Turn on the power pack and observe closely what happens. A piece of white paper held behind the U-tube can help. Make sure the U-tube is kept very still during the experiment.
- Turn off the power as soon as you notice any change at the positive electrode, or when you smell a ‘bleachy, swimming pool’ smell. This will probably take less than five minutes.
Teaching notes
This experiment is an interesting introduction to the electrolysis of brine. It is probably best not used as the first electrolysis that students encounter. They would really struggle to explain for themselves what is going on. It could be followed by the electrolysis of salt solution in industry.
Students should be able to notice bubbles of gas at each electrode. At the positive electrode, the indicator turns red initially, and is then bleached to colourless. This indicates the presence of chlorine. At the negative electrode the indicator turns purple. The remainder of the solution stays green.
The product at the negative electrode is hydrogen. This can be difficult for students to understand.
Some of the water will ionise, that is, turn to hydrogen (H+) and hydroxide (OH–) ions.
When the sodium chloride is dissolved in water, the ions forming the ionic solid separate out. This means that there are actually 4 ions present in the solution: H+, OH–, Na+ and Cl–.
The negative ions are attracted to the positive electrode. The chloride ions are discharged (giving chlorine) in preference to the hydroxide ions. These are left behind in solution.
At the negative electrode, the hydrogen ions are discharged (producing hydrogen gas) in preference to the sodium ions. These are also left behind in solution. Thus sodium hydroxide solution remains. This is the cause of the purple colour of the indicator at the negative electrode.
In time, the green colour of the indicator in the middle would change too, as the ions diffuse through the resulting solution.
Equations
2H+ + 2e–→ H2 [negative electrode, cathode]
2Cl–→ Cl2 + 2e– [positive electrode, anode]
H2 O → H+ + OH–
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
The experiment is also part of the Royal Society of Chemistry’s Continuing Professional Development course: Chemistry for non-specialists.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet