Use this demonstration and class practical to investigate the properties of hydrogen chloride, including its solubility in water
For this session, students begin by observing a demonstration in which the teacher prepares hydrogen chloride gas and uses a fountain experiment to illustrate its solubility in water, forming hydrochloric acid. Students may then investigate some of the properties of hydrochloric acid for themselves, including its reactions with sodium hydroxide, magnesium and marble chips.
Preparation of hydrogen chloride gas on a large scale must be done as a demonstration by a teacher.
Investigating some of the properties of the solution of the gas in water – that is hydrochloric acid – can be done either as a part of the demonstration or as a class experiment, unless the demonstration is only being used to show the great solubility of gases like hydrogen chloride in water.
The demonstration of the fountain experiment should take about 15 minutes and the class investigation of properties of hydrochloric acid about 20 minutes.
Equipment
Apparatus
- Eye protection (including goggles for generating and handling hydrogen chloride gas)
For the fountain demonstration
- Access to a fume cupboard
- Safety screens
- Large round-bottomed flask, 500–1000 cm3 (see note 11 below)
- Bung to fit flask, with holes for a teat pipette (with detachable teat) and delivery tube as shown (see notes 12, 13 and 15)
- Valve, consisting of a short length of rubber tubing and a Mohr’s clip
- Glass trough or plastic bowl
- Short lengths of rubber tubing
- Clamp stands, bosses and clamps (see note 14)
- Small glass funnel, to fit inside a 250 cm3 beaker
- Beaker, 250 cm3
- Conductivity testing apparatus, including:
- 3 V DC power supply
- Graphite electrodes in a bung
- Leads
- Clips
- Torch bulb in holder
For testing the properties of pre-prepared dilute hydrochloric acid
- Test tubes, x4
- Test tube rack
- Plastic dropper pipettes
- Wooden splints
Chemicals
- Concentrated sulfuric acid (CORROSIVE), 10 cm3
- Sodium chloride, 30 g (see note 5 below)
- Universal indicator solution
- Access to the following:
- Dilute hydrochloric acid, 0.1 M
- Sodium hydroxide solution, 0.1 M (IRRITANT)
- Limewater (IRRITANT), fresh, a few cm3
- Universal indicator solution (HIGHLY FLAMMABLE)
- Universal indicator papers
- Magnesium ribbon, 2 cm lengths
- Small marble chips, a few
Health, safety and technical notes
- Read our standard health and safety guidance.
- Work in a fume cupboard and wear goggles for generating and handling hydrogen chloride gas. Wear eye protection for testing the properties of dilute hydrochloric acid.
- Hydrogen chloride gas, HCl(g) is generated (TOXIC, CORROSIVE) – see CLEAPSS Hazcard HC049 and CLEAPSS Recipe Book.
- Concentrated sulfuric acid, H2 SO4 (aq) (CORROSIVE) – see CLEAPSS Hazcard HC098a.
- Sodium chloride, NaCl(s) – see CLEAPSS Hazcard HC047b. Powdered rock salt can be used instead of reagent sodium chloride.
- Dilute hydrochloric acid, HCl(aq) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Universal indicator solution (HIGHLY FLAMMABLE) – see CLEAPSS Hazcard HC032 and CLEAPSS Recipe Book RB000.
- Magnesium ribbon, Mg(s) – see CLEAPSS Hazcard HC059A.
- Limewater, Ca(OH)2 (aq), (treat as IRRITANT) – see CLEAPSS Hazcard HC018 and CLEAPSS Recipe Book RB020.
- Sodium hydroxide solution, NaOH(aq), (IRRITANT) – see CLEAPSS Hazcard HC091a and CLEAPSS Recipe Book RB085.
- The round-bottomed flask should be a thick-walled one made of borosilicate (Pyrex) glass and free of any flaws. The safety screens are necessary, because the sudden vacuum produced when the gas dissolves in water can cause the flask to implode.
- The pipette inserted through the bung should have a removable rubber teat that can be easily attached once the apparatus is in place. It is important to check that the volume of water the teat bulb holds, when filled, is sufficient to ensure that drops of water emerge into the flask, when the teat is squeezed. It helps to fill the teat bulb with water beforehand, and place it upright in a small beaker ready to use.
- The flask and the bung carrying the glass tubes should be dried in an oven at about 80 °C before the experiment.
- It is very important to set up the necessary stand, bosses and clamps, safety screens and the empty trough/bowl in their correct positions on the bench in front of the class before the demonstration. Use the empty round-bottomed flask fitted with the bung and tubes (but no teat bulb on the pipette) to adjust the position of the clamps, so that the long tube and valve reaches almost to the bottom of the bowl. Make sure the flask will not topple over when it fills with water from the bowl. Do this by positioning the bowl on the base of the stand, or putting some extra weights on it.
- The set-up of the hydrogen chloride fountain demonstration can be seen here:
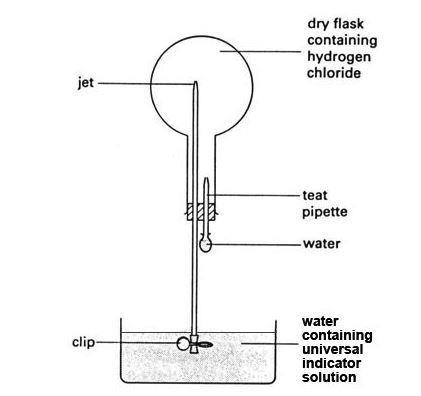
Procedure
Preparing hydrogen chloride and demonstrating its solubility in water (using the fountain experiment)
- Fill the bowl with tap water and add enough universal indicator solution to make the colour clearly visible.
- Set up the hydrogen chloride gas generator in a fume cupboard. Remove the empty round-bottomed flask from its position on the bench set-up to the fume cupboard for filling. Clamp the flask in position near the gas generator.
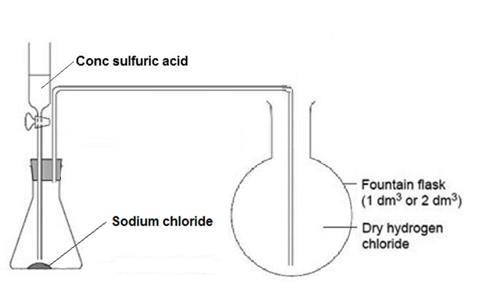
- Purge the air out of the flask containing sodium chloride, by dripping about 1 cm3 of sulfuric acid onto the sodium chloride. A piece of damp universal indicator or blue litmus paper held near the end of the delivery tube turns red when hydrogen chloride starts to emerge from it.
- Position the delivery tube in the round-bottomed flask. Slowly fill the flask with hydrogen chloride, by dripping more sulfuric acid onto the sodium chloride in the gas generator. Test to see when it is full, by holding damp indicator paper near the mouth of the flask. Allow the gas to pass for a little longer, even if the indicator paper turns red.
- Stop the gas stream and remove the delivery tube from the round-bottomed flask. Refit the bung to the flask and clamp it in position over the bowl of water ready for the fountain demonstration. Ensure the valve at the end of the glass tube jet is well immersed in the water and the system is air tight.
- Carefully attach the bulb of the teat pipette filled with water to the pipette tube, without squeezing any water into the flask.
- Remove the clip from the valve. The water starts to rise slowly up the tube as the gas dissolves in it. To speed up this process, squeeze a few drops of water into the flask from the pipette.
- All the hydrogen chloride in the flask dissolves in the water drops from the pipette, creating a vacuum in the flask. Water is then sucked up at great speed from the bowl, creating a spectacular ‘fountain’ in the flask. The indicator in the water changes colour to red. The extent to which the flask fills with water depends on how much air was left in the flask. It can fill almost completely.
Properties of hydrochloric acid
If these experiments are only demonstrated, then hydrochloric acid prepared by bubbling hydrogen chloride from the generator can be used in a fume cupboard. This must be done using the method described below in steps 1–3 because of the great solubility of the gas. If students are to carry out tests on the properties, they should begin at step 4, using pre-prepared dilute hydrochloric acid.
- Half-fill a 250 cm3 beaker with distilled or deionised water. Connect the small funnel to the gas generator with a length of rubber tubing. Clamp it upside down in the beaker, so that the funnel rim is just below the surface of the water. See the diagram below:
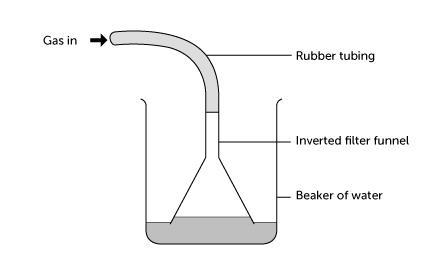
- Bubble hydrogen chloride gas slowly through the funnel, by dripping sulfuric acid onto the sodium chloride. If the gas flow rate is too slow, the water level rises in the funnel and falls in the beaker – until bubbles of air are sucked into funnel. This stops the water rising further. If this happens, increase the gas flow rate.
- After one to two minutes raise the funnel from the beaker and stop the gas flow. Use the conductivity tester to test the conductivity of the solution in the beaker. The lamp should light, showing the presence of ions in solution. If not, bubble more hydrogen chloride through the water and test again.
- Fill three test test tubes to a level of one-third with the hydrochloric acid prepared above or use dilute hydrochloric acid. Add a couple of drops of universal indicator solution to one of the tubes. It turns red, indicating the presence of a strong acid.
- Add sodium hydroxide solution slowly to the same test tube using a pipette. Shake to mix after each addition. Continue adding until the alkali neutralises the acid and the colour changes from red, through yellow-green, to purple.
- Add a strip of magnesium to a second test tube of acid. It fizzes vigorously, giving off a colourless gas, and dissolves to give a colourless solution of magnesium chloride. The gas can be collected by holding an inverted test tube over the reaction for a several seconds. Test for hydrogen by applying a flame to the mouth of the inverted tube. You should hear a squeaky pop should.
- Add a few marble chips to a third test tube of acid. They fizz, giving off a colourless gas – carbon dioxide. The presence of this gas can be proved using the limewater test.
Teaching notes
It advisable to try the fountain experiment before demonstrating it to a class, to make sure the flask can be successfully filled with HCl gas. Be careful not to disconnect the flask from the gas supply too soon, even if HCl can be detected at the outlet. Oven drying the glass apparatus beforehand improves the chances of success.
The solubility of hydrogen chloride in one volume of water at 0 °C and 1 atm is 506 volumes.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet