The distinctions between pure substances and mixtures, and between elements and compounds are fundamental in chemistry
The materials comprise a pre-test, a study activity, and a post-test.
Pre-test questions
- An element is…
- A compound is…
- A mixture is…
You will find six diagrams showing the particles in some samples of materials.
Each diagram is meant to show either an element, a compound or a mixture. Decide whether each diagram represents an element, a compound, or a mixture, and explain your reasons.
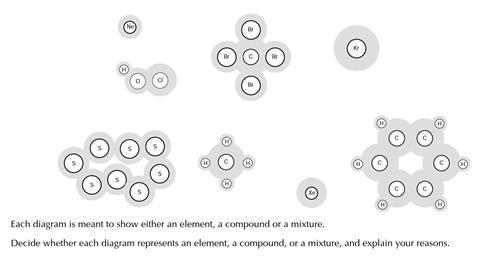
Pre-test answers
Various definitions of elements and compounds may be used. The following is suggested. Atom or nucleus can be used instead of core.
- An element is a pure substance which contains identical atoms or molecules with only one type of atomic core.
- A compound is a pure substance which contains identical molecules with two or more types of atomic core.
- A mixture is a material which has two or more types of molecules. Clearly, these definitions only apply to molecular materials, and will need to be extended to ionic and metallic materials later.
- Compound - one type of molecule; each molecule has more than one type of atomic core.
- Element – one type of atom or molecule, with only one type of atomic core.
- Element – one type of atom or molecule, with only one type of atomic core.
- Mixture (of two compounds) – two types of molecule present.
- Mixture (of two elements) – two types of atom present.
- Compound - one type of molecule; each molecule has more than one type of atomic core.
Study task questions
Pure substances and mixtures
In science, it is important to know the difference between pure substances and mixtures of several substances. Scientists think about the differences in terms of the particles which are in the materials.
Scientists believe that all matter (all solids, liquids and gases) is made up from tiny particles that are much too small to be seen. The tiniest particles are given names like ‘electron’ and ‘proton’.
These are arranged into slightly larger (but still very tiny) particles called atoms, ions and molecules. In many materials, the particles are called molecules. Here are some pictures that scientists use to represent atoms and molecules.
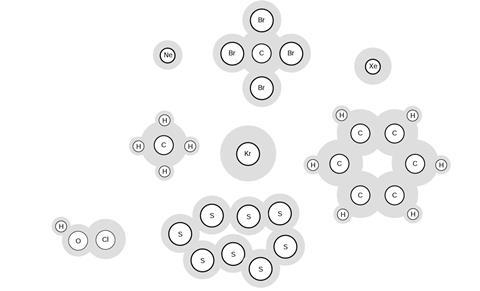
The letters are labels used by scientists to help identify the particles. There are many different types of atoms and molecules, and these pictures just show a few examples.
When substances are mixed, their molecules become mixed up. We call the new material a mixture. A mixture contains more than one type of atom or molecule. The following diagrams show the molecules in two pure substances before mixing, and the mixture of molecules afterwards.
Look at the diagrams closely, and label each of them as either a single substance, or a mixture.
Study task answers
- Single substance
- Single substance
- Mixture
- Mixture … molecule
- Single substance … molecule
- Compound … [atomic] core [or atom or nucleus] …
- Element … [atomic] core [or atom or nucleus] …
- Compound
- Mixture
- Element
Post test questions
Each of the following diagrams show the particles in a material. For each diagram, write whether you think it represents an element, a compound or a mixture
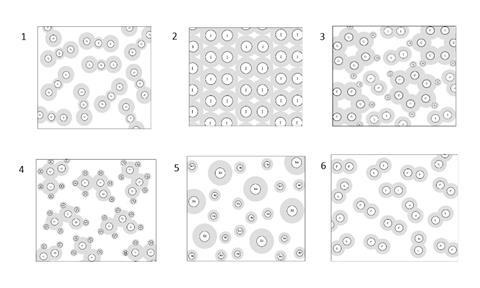
Post test answers
- Compound
- Element
- Mixture
- Compound
- Mixture
- Element
Notes
For the full version of this chapter, see downloads below.
Downloads
Elements, compounds and mixtures
PDF, Size 1.29 mb
Websites
Additional information
These resources have been taken from the book, Chemical Misconceptions : Prevention, diagnosis and care: Theoretical background, Volume 2, by Keith Taber.

Chemical misconceptions
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
 Currently reading
Currently readingElements, compounds and mixtures
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35























































1 Reader's comment