This article looks at detecting strep throat bacterium using touch spray mass spectrometry. It will help you understand the research the journal article is based on, and how to read and understand journal articles. The research article was originally published in our Analyst journal.
Strep throat spotted in seconds
Detection of strep throat bacterium directly from medical swabs by touch spray–mass spectrometry
Click here for the full article in the journal Analyst
Click here for the article in Chemistry World
Authors: Alan K. Jarmusch, Valentina Pirro, Kevin S. Kerian, and R. Graham Cooks
Why is this study important?
Strep throat is a common illness that is diagnosed millions of times annually in the United States alone.1,2 One bacterium, Streptococcus pyogenes or group A streptococcus (GAS), is responsible for the vast majority of cases. Diagnosis is crucial in children, elderly patients, and in regions in which rheumatic and scarlet fever are prevalent because life-threatening complications are possible. Clinical symptoms like sore throat and fever are not exclusive to S. pyogenes or strep throat making it hard to diagnose.1 Rapid strep tests are currently used for point-of-care diagnosis (ie in a doctor’s office), as shown in Figure 1A. The patient’s throat is swabbed and the swab inserted into the test receptacle, which functions similarly to a common pregnancy test. The test provides a binary (yes/no) answer on the presence/absence of S. pyogenes. Although the rapid test is easy to use and provides a straightforward outcome, the results are not reliable. Accordingly, alternative but simple methods to detect the bacteria responsible for infection (with increased confidence) are desirable, with the intention of improving patient care.
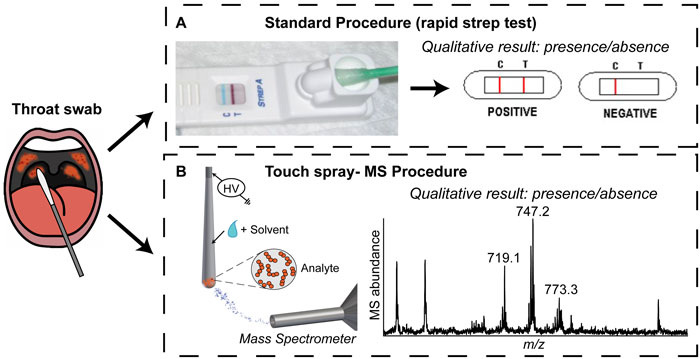
Figure 1. Strep throat diagnosis: (A) schematic of the standard procedure and (B) TS-MS procedure described in Jarmusch et al. Analyst (2014) 139: 4785-4789.3
Mass spectrometry (MS) is a powerful analytical technique capable of detecting molecules, such as lipids, in complex matrices. Lipids provide cellular structure and contribute to cell function.4 Lipid profiles, unique patterns of lipids and their amount, are characteristic of cell type and are specific enough to allow discrimination between different bacterial species.5,6 Therefore, lipid profiling by MS represents a possible alternative strategy for S. pyogenes detection, illustrated in Figure 1B.
Further information
- A review paper on 'Ambient ionization mass spectrometry: current understanding of mechanistic theory; analytical performance and application areas'
- A review paper on 'Bacterial identification: from the agar plate to the mass spectrometer'
- Selected further reading from RSC books:
- Detection Challenges in Clinical Diagnostics
- Analysis by Electrospray Mass Spectrometry
- Biomarker Discovery for Clinical Application
- Miniaturization and Mass Spectrometry
What is the objective?
The objective was to develop a MS procedure for the detection of S. pyogenes, the primary pathogen of strep throat. The authors intended to adapt an ambient ionization method that would allow for direct detection of S.pyogenes from the throat swabs. Ambient ionization allows the generation of ions under atmospheric conditions (pressure, temperature, etc) and requires minimal sample preparation.7 Touch spray (TS) was used because sampling and ionization occur from the same substrate.8 In addition to this main objective, the authors' aim was also to create a method capable of differentiating multiple bacteria using a single method.
What was the overall plan?
Test medical swabs using touch spray-mass spectrometry
Detect S. pyogenes from culture
Compare the spectra from two related bacteria
Detect S. pyogenes in simulated clinical samples
What was the procedure?
Test medical swabs using touch spray-mass spectrometry (TS-MS)
TS-MS using swabs is performed by positioning the swab in front of the mass spectrometer (Figure 1B). Solvent is applied to the swab and then a high voltage is applied, generating a strong electric field that results in the emission of analyte-containing charged droplets. The initial step in the development of a TS experiment with swabs was to test many (and various) swabs in order to find those most suitable for the generation of ions for MS. Medical swabs are commonly used for sampling orifices and body surfaces with their specific construction based on application. Most medical swabs are comprised of a paper or plastic handle and a rounded adsorbent tip (for example cotton). The swab that performed best was designed for sampling the oral cavity, specifically the pharynx, and was made of rayon with an aluminium wire handle. This worked best (based on signal intensity and lowest background) for three major reasons: (1) the metallic handle of the swab allowed voltage to be easily applied and transferred to the swab, (2) the swab is a porous polymer from which ions are readily generated,9 and (3) the shape of the swab; rounded but coming to a point, which increased the electric field to allow ionization. A second step in the method development was to optimize spray stability and signal intensity. A vertical orientation of the swabs relative to the mass spectrometer was found to provide the best reproducibly. Different solvents for lipid extraction were tested to obtain reproducible lipid information. A single addiction of methanol (~40 µL) onto the swab tip was the final condition chosen.
Detect S. pyogenes from culture
After the swab and experimental conditions were selected, the next step was to test if the bacterium responsible for strep throat, S. pyogenes, could be detected from the swab. The authors cultured S. pyogenes on blood agar and then sampled about 20 micrograms of material from a single colony, a group of individual organisms living in close proximity, using the rayon tip of the medical swab. The swab was then put near the mass spectrometer and held vertically with a ring-stand and clamp. Touch spray ionization was performed, as described above, resulting in the spectrum shown in Figure 2. Negative ions generated from the swab appear as peaks in the spectrum. The peaks at approximately m/z 700-800 are identified as lipids that originate from the bacteria.
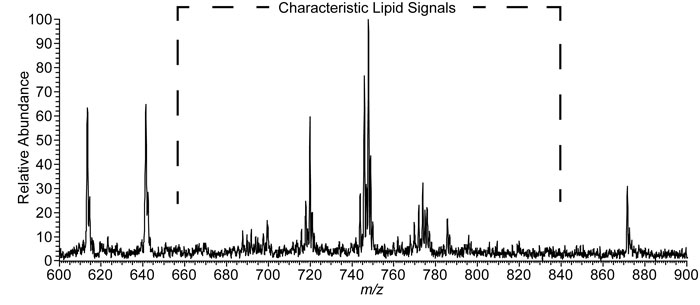
Figure 2. Negative ionization mode TS-MS spectra of a single colony of S. pyogenes sampled from culture.
Compare the spectra from two related bacteria
There are many different types of bacteria that can cause strep throat, or more broadly bacterial infection of the pharynx. The authors aimed to develop a method that was able to differentiate multiple causes (differential diagnosis) in a single test, not currently possible in commercial rapid strep tests. The ability to differentiate two similar species in the same genus was tested in vitro. TS-MS spectra resulting from S. pyogenes and Streptococcus agalactiae, group B streptococcus (GBS), are displayed in Figure 3. The lipid profiles of the two Streptococcus spp. are visibly different in the m/z 600-800 region. It turns out that these patterns are characteristic of the bacteria, so detailed MS interpretation is not needed: pattern recognition allows diagnosis. The ability of TS-MS to reproducibly detect differences in the lipid profile of bacteria possessing similar culturing features (for example beta-hemolysis or the breakdown of red blood cells resulting in transparency) is notable, while the ability to differentiate Streptococcus spp. is highly clinically relevant. The visual differentiation of S. agalactiae serves to illustrate the capability for differential diagnosis from a single testing methodology. It also simultaneously presents another possible and independent application of this methodology, as rapid GBS detection, which is sought-after in neonatal care.10
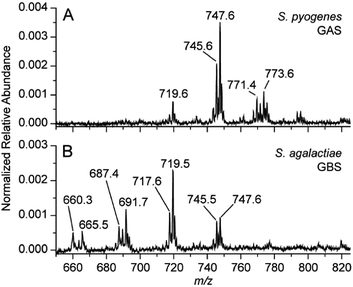
Figure 3. Negative mode TS-MS spectra of S. pyogenes (A) and S. agalactiae (B) sampled using a single colony from culture. Annotated peaks are tentatively identified as bacterial lipids but the spectral pattern rather than individual components is used to recognize the microorganism.
Detect S. pyogenes in simulated clinical samples
The detection of S. pyogenes lipids was tested with a simulated clinical sample. Human oral fluid, saliva and cheek epithelial cells were used to test the method. The swab was dipped into ~1mL of human oral fluid, absorbing an estimated 40 microliters, and then subsequently used to sample a single colony of S. pyogenes from culture - simulating a clinical throat swab. Touch spray ionization was performed resulting in the detection of multiple ions attributed to human and bacterial components. The lipid region (m/z 700-900), Figure 4, contained ions corresponding to bacterial lipids. Bacterial lipids m/z 719.5 and 773.6 were similar in relative abundance to those seen in in vitro experiments whereas m/z 745.5 and 747.6 differed in pattern. The ions m/z 719.5 and 773.6 were only detected when S. pyogenes was present.
.gif)
Figure 4. Negative ionization mode TS-MS spectrum displaying phospholipid ions corresponding to bacterial and human components of a throat swab.
What are the conclusions?
This study details the initial development of a new non-invasive diagnostic technique, directed towards a specific clinical condition (strep throat). It addresses the problem of diagnosis of bacterial infections at the point-of-care. TS-MS performed using medical swabs allowed the rapid analysis of S. pyogenes, and it required only seconds to obtain the test data. Detection of bacterial lipids originating from S. pyogenes was performed in vitro and from simulated throat swabs using a single colony. In vitro experimentation provided visual differentiation of S. pyogenes and S. agalactiae.
What are the next steps?
A clinical trial is required to test this method and this is now being planned. The future development of swab design and materials should facilitate MS analysis, improving performance including reproducibility, background reduction and improved signal levels. Additionally, extensions of the method to other microorganisms and the ability to detect bacterial and human lipids concurrently in clinical samples might provide additional data pertinent to disease. Since MS allows detection of small molecules, such as illicit drugs and pharmaceuticals, with extremely high sensitivity and specificity, the application of TS-MS experiments with medical swabs for oral fluid analysis is envisioned to be of great benefit for therapeutic drug monitoring, drug testing, etc. Indeed, switching from drug detection in blood to oral fluid is the current trend in several clinical and forensic applications.11,12
Abbreviations used in text
Abbreviation | Name | Role | Links |
GAS | Group A streptococcus | Lancefield group A Streptococci defined by bacterial carbohydrates, abbreviation GAS is commonly used in medicine, includes Streptococcus pyogenes. | |
GBS | Group B streptococcus | Lancefield group B, commonly referred to as GBS in medicine, includes Streptococcus agalactiae. | |
MS | Mass Spectrometry | Instrumental analysis technique that measures the mass-to-charge of gas-phase ions using a mass spectrometer. The information obtained is indicative of mass and quantity of molecules in a sample. | |
S. Pyogenes | Streptococcus pyogenes bacterium | A species of Streptococci that is the primary cause of strep throat and other infections. | |
TS | Touch Spray | A method of sampling and ionization using the same substrate. | |
TS-MS | Touch spray - mass spectrometry | A hyphenated ambient mass spectrometry method in which ions are created using touch spray and then analyzed using a mass spectrometer. |
Alan Jarmusch, one of the authors, answers some additional questions on his group's work.
Could you please summarise how Strep throat is currently diagnosed and explain how the system you have developed is better than those that are already available?
Strep throat is primarily diagnosed using a patient’s clinical symptoms but nearly always aided by rapid tests performed during a visit to the physician. The rapid tests (called rapid antigen detection tests – RADT) are necessary in diagnosing strep throat as the common symptoms (sore throat, fever, etc) are not unique to only strep throat. The rapid test takes about 15-20 minutes, depending on the commercial product, and gives a visual indication of the test result – similar to an at-house pregnancy test.
Our methodology aims to address gaps and expand capability, (1) we hope to match or improve diagnosis when a patient has an infection (true positive) while improving on the false negative rate, thereby improving patient care. One way we accomplish this is by using many biomolecules (specifically lipids - they are part of all bacteria cells (and our cells as well)) which are detected simultaneously and used in combination for diagnosis. (2) Our hypothesis is that (in specific circumstances) the lipids detected from bacteria allow for them to be distinguished. We hope to answer to the question, “what (if any) bacteria is present?” which should allow physicians to better treat patients.
How did the idea for the design of your system come about? What was the inspiration behind the study?
The idea originated during development of touch spray ionization which was itself motivated by a need for cancer diagnosis during tumor removal operations. The original probe used in those experiments was metallic and sharp, not practical for use in strep throat diagnosis – it was too harsh for someone’s throat. With the idea of translating touch spray ionization into a non-invasive procedure that patients could withstand in the doctor’s office we started experimenting with swabs.
The inspiration is quite personal to the author, Alan Jarmusch, who was afflicted with strep throat as a child (like many); however, his case progressed beyond most. A fairly minor case of rheumatic fever, left the author unable to walk and subjected to a sting of doctor visits and antibiotic regiments.
Could you briefly explain in simple terms how your system works. How do you get from a throat swab to a read out of whether or not you have Strep throat?
The simple answer is that we are detecting the molecules from bacteria directly from the throat swab. Mass spectrometry, the method of detection, requires ions, our methodology generates ions directly from the swab.
The read out of our mass spectrometry method is complex in its unrefined form called a spectrum (spectra plural), containing peaks which correspond to one or more ions. Currently, the read out relies only on visual pattern recognition. However, future development will include a method to automatically recognize the peaks and provide an easily read output – could be as simple as red-light, “you have strep throat” or green-light, “you do not have strep throat.”
Could you describe any problems you had during this research. Which part of the work towards this paper proved to be most challenging and how did you overcome the problems that you faced?
One major obstacle was adapting a medical device (swab) for mass spectrometry, something it was not intended. The shape and materials of the swab were critical in how well it performed this specific task. A number of different swabs were tested and the best one was pursued. The performance of the swab for MS could certainly be improved by small changes in materials and manufacturing.
How can you see the system that you have developed being used in the future? Do you expect that your system will one day become commercially available? What needs to be done before this can be achieved? What other uses do you see for your system?
The prospective application would be for point-of-care (in the physician’s office) diagnosis of strep throat and additional diseases that are tested using swabs. The increasing development of miniature mass spectrometers will certainly aid in this development allowing laboratory scale instruments, too large for most doctor’s offices, to be adapted into compact clinical instruments.
We hope that this system would become commercially available after some critical development steps. Work needs to be done to extend our method to true clinical samples, improving the methodology further, and eventually comparing our method to that or current methods. The extension of this method to additional bacteria is foreseeable as well as other common medical test that would benefit patient care done at the point-of-care.
What are the limitations of the technique you have developed? What is the next step to overcome these limitations?
Many of the limitations stem from the inexperience in using medical devices for mass spectrometry, they are not created for such a purpose and are only a number of previous examples. Overcoming the limitations should be a matter of developing a mass spectrometry-friendly swab. Another limitation is the high initial cost of a mass spectrometer and the expertise need to run such an instrument; however, future full-size and miniature mass spectrometers are likely to address these issues.
What are your plans now? What are you doing at the moment and what will you be working on in the near future?
We are planning to develop the method so that is more rigorous, extend the methodology to incorporate additional bacteria, and test our methods using real clinical samples.
The authors

Alan K. Jarmusch
Alan K. Jarmusch received a B.S. in Biochemistry from the University of North Carolina at Greensboro and is currently a Ph.D. candidate in Chemistry at Purdue University, mentored by Professor R. Graham Cooks. His current research interest includes the development and application of ambient ionization-mass spectrometry in the fields of medicine and natural products.

Valentina Pirro
Valentina Pirro received her Ph.D. in Science and High Technology from the University of Turin, Italy. She is currently a postdoctoral researcher in Chemistry at Purdue University. Her current research interest includes the application of cutting-edge mass spectrometric techniques (e.g., ambient mass spectrometry, imaging mass spectrometry) in the field of clinical and forensic toxicology.

Kevin S. Kerian
Kevin S. Kerian received a B.S. in Chemistry and Biochemistry from Western Kentucky University and is currently in the PhD program at Purdue University, studying analytical chemistry under the advisement of Professor R. Graham Cooks. His current research interest pursues advancing translation science through the development and application of ambient ionization-mass spectrometry for molecular diagnostics.

Graham Cooks
Graham Cooks was educated at the University of Natal, South Africa, and at Cambridge University in the UK. Since 1990 he has been Henry Bohn Hass Distinguished Professor of Chemistry at Purdue University. Cooks has been a major contributor to advances in instrumentation and methodology for mass spectrometry. His interests involve construction of mass spectrometers and their use in fundamental studies and applications.
References
1. Gieseker, K. E., Roe, M. H., MacKenzie, T. & Todd, J. K. Evaluating the American Academy of Pediatrics diagnostic standard for Streptococcus pyogenes pharyngitis: backup culture versus repeat rapid antigen testing. Pediatrics111, e666-e670 (2003).
2. Clerc, O. & Greub, G. Routine use of point‐of‐care tests: usefulness and application in clinical microbiology. Clinical Microbiology and Infection16, 1054-1061 (2010).
3. Jarmusch, A. K., Pirro, V., Kerian, K. S. & Cooks, R. G. Detection of strep throat causing bacterium directly from medical swabs by touch spray-mass spectrometry. Analyst139, 4785-4789 (2014).
4. Eberlin, L. S., Ferreira, C. R., Dill, A. L., Ifa, D. R. & Cooks, R. G. Desorption electrospray ionization mass spectrometry for lipid characterization and biological tissue imaging. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids1811, 946-960 (2011).
5. Hamid, A. M., Jarmusch, A. K., Pirro, V., Pincus, D. H., Clay, B. G., Gervasi, G. & Cooks, R.G. Rapid Discrimination of Bacteria by Paper Spray Mass Spectrometry. Analytical chemistry (2014).
6. Sauer, S. & Kliem, M. Mass spectrometry tools for the classification and identification of bacteria. Nature Reviews Microbiology8, 74-82 (2010).
7. Badu-Tawiah, A. K., Eberlin, L. S., Ouyang, Z. & Cooks, R. G. Chemical aspects of the extractive methods of ambient ionization mass spectrometry. Annual review of physical chemistry64, 481-505 (2013).
8. Kerian, K. S., Jarmusch, A. K. & Cooks, R. G. Touch spray mass spectrometry for in situ analysis of complex samples. Analyst (2014).
9. Liu, J., Wang, H., Manicke, N. E., Lin, J.-M., Cooks, R. G. & Z. Ouyang. Development, characterization, and application of paper spray ionization. Analytical chemistry82, 2463-2471 (2010).
10. Bergeron, M. G. et al. Rapid Detection of Group B Streptococci in Pregnant Women at Delivery. New England Journal of Medicine343, 175-179, doi:doi:10.1056/NEJM200007203430303 (2000).
11. Bosker, W. M. & Huestis, M. A. Oral fluid testing for drugs of abuse. Clinical chemistry55, 1910-1931 (2009).
12. Di Corcia, D. et al. Determination of pharmaceutical and illicit drugs in oral fluid by ultra-high performance liquid chromatography-tandem mass spectrometry. Journal of Chromatography B (2013).
Additional information
Journal articles made easy are journal articles from a range of Royal Society of Chemistry journals that have been re-written into a standard, accessible format. They contain links to the associated Chemistry World article, ChemSpider entries, related journal articles, books and Learn Chemistry resources such as videos of techniques, and resources on theory and activities. They should facilitate students understanding of scientific journal articles and how to extract and interpret the information in them.


















No comments yet