Measure the volume and the mass of a gas from a pressurised cylinder by collecting the gas over water and calculating its relative molecular mass.
In this experiment, students observe as a sample of butane from a small pressurised cylinder is collected over water in a measuring cylinder. By measuring the volume of the gas, and determining its mass by weighing the cylinder before and after collection, students can calculate the relative molecular mass of the butane.
This is most likely to be done as a teacher demonstration. Teachers of advanced students may wish to consider the possibility of a student practical, but would need to carry out very careful risk assessments in the context of the capabilities of their students.
Although the most convenient gas for this is butane, other gases may be available in similar small cylinders.
The collection of a gas sample, and the weighing of the gas cylinder before and after this, should take about 5–10 minutes as a demonstration.
Equipment
Apparatus
- Eye protection
- Access to a fume cupboard (see note 4 below)
- Measuring cylinder (1 dm3)
- Stand and clamp (see note 5)
- Trough (see note 6)
- Delivery tube, flexible and gas-tight (see note 7)
- Top-pan balance (see note 8)
Optional
- Thermometer to measure room temperature +/– 0.5 °C, with digital display if available
- Access to an accurate measurement of atmospheric pressure
Chemicals
- Gases available in small pressurised cylinders, for example (see note 9 below):
- Butane in lighter refill cans, from a camping gas stove or a gas blow lamp
- Propellant gas from aerosol cans (this could be turned into an investigation into which gas is being used as the propellant)
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout. Remove all possible sources of ignition. Ensure adequate room ventilation.
- Butane, C4 H10 (g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC045a.
- A fume cupboard should be used if it is difficult to attach the delivery tube or control the valve.
- The stand and clamp should be able to hold the large measuring cylinder full of water securely.
- The trough should be sufficiently large to allow the easy immersion of the lower end of the upturned measuring cylinder filled with water, and then allow the displacement of 1 dm3 of water.
- The flexible delivery tube must be long enough to permit easy manipulation for the gas collection. Make sure the delivery tube will connect securely to the canister of gas. The precise way in which the delivery tube may be attached to the pressurised cylinder of butane will depend on the cylinder involved, and the design of its valve; some ingenuity may be required. Some types do NOT reseal. The connection must be gas-tight and secure. Lighter refill cans should make a simple connection to tubing of appropriate diameter. Removing the burner from camping gas stoves and blowlamps should enable the tubing to be connected directly above the valve.
- A top-pan balance accurate to +/– 0.01g is sufficient, but +/– 0.001g would be ideal, with (if available) output to a computer to display the reading.
- In rural areas the laboratory gas supply may be butane or propane.
Procedure
- Before the lesson, fill the measuring cylinder with water to the brim, close the mouth of the cylinder firmly with the palm of the hand, and invert the cylinder into the trough of water. Clamp the cylinder firmly, allowing sufficient room under the cylinder mouth to insert the end of the delivery tube.
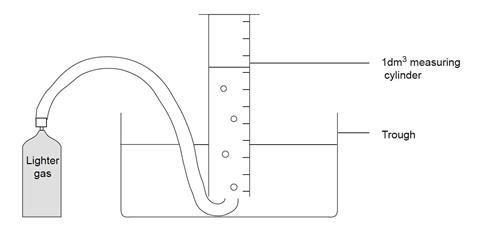
- Weigh the gas canister.
- Connect the delivery tube to the canister. Place and hold the other end of the delivery tube under the mouth of the inverted measuring cylinder.
- Carefully open the valve of the canister and collect exactly 1 dm3 of gas, ensuring the water levels inside and outside the cylinder are the same at the end. Close the valve.
- Disconnect the delivery tube from the gas canister and dry the outside of the canister thoroughly, if necessary, and reweigh.
- Release the gas from the measuring cylinder with due regard to safety, remembering it is significantly denser than air – preferably in the fume cupboard, or out of a window.
- Record room temperature and pressure if required (see the teaching notes below for reference to pV = nRT calculations).
Teaching notes
There are two possible routes for using the results of the experiment to calculate the RMM:
- One mole of a gas, irrespective of its chemical nature, occupies approximately 24 dm3 at standard room temperature and pressure. The experiment has established the mass of 1 dm3, so the mass of 1 mole is simply 24 times that mass.
- For a more accurate analysis, if the class has already studied the ideal gas equation, they can use the relation: pV = nRT to calculate the number of moles in 1 dm3. This requires the records of room temperature and pressure at the time of the experiment. From this the mass of one mole can be calculated.
Investigating the nature of the propellants in aerosol cans is essentially an extension of this experiment. The composition of most present-day aerosol propellants is a mixture of butane isomers and propane. The varying proportions of propane and butane will lead to a measured RMM between those of propane (46) and butane (58). By measuring the apparent RMM, the proportions of propane and butane can be calculated. However, the other ingredients of the aerosol may affect the measurements.
Oxygen (oxidising) is also available in small pressurised canisters – see CLEAPSS Hazcard HC069. Teachers adapting this experiment for measuring the RMM of oxygen will need to consult their employer’s risk assessments, but this may be a preferred alternative to butane for use as a class experiment. However, the purity of the oxygen in these cylinders may be significantly less than 100%, which will affect the RMM value obtained.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet