Show how dyeing involves chemical interactions between dyes and the molecular nature of different fibres in this demonstration
In this experiment, students observe as samples of different fabrics are placed in a single dye bath containing three dyes – one blue, one yellow and one red. The materials emerge dyed different colours, illustrating the fact that dyeing depends on specific chemical interactions between the dyes and fibres used.
This is likely to be performed as a teacher demonstration, in the context of the topic of organic dyestuffs, either as an introductory attention-grabber, or later in the topic to stimulate discussion of the chemical interactions involved in dyeing. If the materials for the demonstration have been fully prepared, the demonstration itself should take around 30 minutes. The demonstration can be scaled up as required for larger audiences.
Equipment
Apparatus
- Eye protection
- Beakers, 400 cm3, x4
- Large watch glasses or Petri dishes, x4
- Tongs or forceps
- Scissors
- Sample tubes, stoppered, x6
- Bunsen burner
- Tripod
- Gauze
- Heat resistant mat
- String and paper clips, crocodile clips or clothes pegs (see note 5 below)
- Samples of these fabrics in white (see note 6), such as:
- Wool
- Silk
- Nylon
- Cotton
- Polyester
- Cellulose acetate (‘triacetate’)
- Polyester/cotton mix
- Access to top pan balance
Chemicals
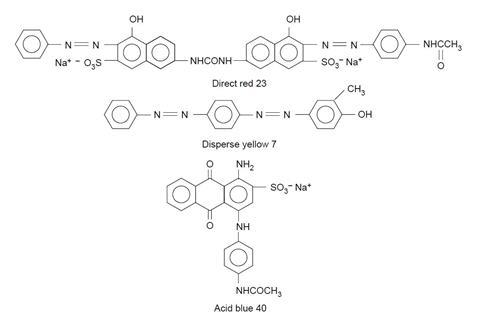
- Acid blue 40, 0.06 g
- Disperse yellow 7, 0.04 g (IRRITANT)
- Direct red 23, 0.04 g (IRRITANT)
- Hydrochloric acid, 2 M (IRRITANT), small quantity
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Acid blue 40, Disperse yellow 7 (IRRITANT) and Direct red 23 (IRRITANT) – see CLEAPSS Hazcard HC032. Bottles should be opened in a fume cupboard. The dilute solutions are low hazard but will stain skin and clothes. Protective gloves (preferably nitrile) should be worn. Weigh out into stoppered sample tubes, two samples of 0.02 g of each of the red and yellow dyes, and two samples of 0.03 g of the blue dye. Label each sample tube. These dyes are available from Philip Harris Ltd or from Kemtex Educational Supplies. If the teacher requires the dye bath to be made ready before the lesson, dissolve 0.02 g of each of the red and yellow dyes, and 0.03 g of the blue dye in 200 cm3 of water in a beaker, add a few drops of dilute hydrochloric acid and heat to boiling.
- Hydrochloric acid, HCl(aq) (IRRITANT at concentration used) – see CLEAPSS Hazcard HC047a and CLEAPSS Recipe Book RB043.
- Prepare a ‘clothes line’ using string between laboratory stands, long enough and with enough ‘pegs’ (crocodile clips, paper clips or miniature clothes pegs) to hang out the dyed fabric samples.
- About 100 cm2 of each fabric, or a few cm of thread will be sufficient. As a minimum, samples of cotton, either polyester or cellulose acetate, and either wool, silk, or nylon are required. White nylon, 100%, can be difficult to obtain and it may be necessary to try a second-hand clothes shop. Cut four strips of each fabric (about 4 cm x 4 cm is suitable). Each fabric should be easily identifiable in some way, for example by cutting different shapes.
Procedure
- If the dye bath has yet to be prepared, dissolve 0.02 g of each of the red and yellow dyes and 0.03 g of the blue dye in 200 cm3 of water in a beaker, add a few drops of dilute hydrochloric acid and heat to boiling.
- Place a sample of cotton, cellulose acetate and either wool, silk or nylon in the dye bath and simmer gently for about 10 minutes.
- Remove the fabrics with forceps or tongs, rinse under running water, and hang up on the ‘clothes line’. Cotton will be dyed red, acetate yellow and wool, silk or nylon blue-green. (Some of the yellow direct dye will take to these materials as well as the blue acid dye.)
- Try other materials as well if desired. Polyester will be dyed yellow and polyester/cotton will become orange.
- Now examine the effect of the dyes individually. Make three dye baths, the first containing 0.02 g of red dye in 200 cm3 of water, the second containing 0.02 g of the yellow dye in 200 cm3 of water and the third containing 0.03 g of the blue dye in 200 cm3 of water. Add a couple of drops of hydrochloric acid to each dye bath and heat to boiling.
- Place a sample of each fabric in each dye bath and treat as before, ie simmer for 10 minutes, remove the samples and rinse. Typical results are shown in the table.
| Dyes | Silk | Wool | Nylon | Cotton | Acetate | Polyester | Polycotton |
|---|---|---|---|---|---|---|---|
| Mix | olive-green | olive-green | olive-green | red | yellow | yellow | orange |
| Red | pale orange-red | pale orange-red | pale orange-red | red | almost white | pink | pink |
| Blue | blue | blue | blue | very pale blue | white | white | almost white |
| Yellow | orange | orange | orange | pale yellow | bright yellow | bright yellow | bright yellow |
Teaching notes
Different dyes bond to fabrics in different ways.
Acid dyes contain acidic –CO2H and –SO3H groups which bond to the basic –NH groups in the amide linkages of wool, silk and nylon.
Direct dyes bond by hydrogen bonding and take well to cellulose-based fibres such as cotton, viscose and rayon which have many –OH groups.
Disperse dyes are not water-soluble. They exist in the dye-bath as a fine suspension (hence the name), and are absorbed as a solid solution by hydrophobic fabrics such as polyesters.
After the demonstration, students who have already studied the chemical structures of different types of fibre could be asked to predict the effects on other fabrics, for example on silk and nylon, which are polyamides like wool. They could also be asked to predict the effect of the dyes on a mixed fabric, such as cotton-polyester. They may now be able to offer a possible explanation for some odd effects in washing machine accidents, where labels and trim may emerge a different colour to the rest of the garment.
This demonstration could be followed up by various investigations, for example:
- Devising mixed dye-baths to produce different colours to the ones demonstrated using the chemical principles fabrics described above.
- Investigating the effect on the colours produced in different fabrics of:
- Mordants such as salt or alum – see CLEAPSS Hazcards HC047b and HC002B
- pH of the dye bath
- Time in the dye bath
- Temperature of the dye bath
- How fast (resistant to washing out) are the dyes to a variety of treatments?
Further information
- A good account of which types of dyes dye which fabrics is given in The essential chemical industry, p 42. University of York: The Chemical Industry Education Centre, 1989.
- Kemtex Educational Supplies provide a range of information sheets about dyes and dyeing, and related processes such as transfer printing, as well as supplying a wide range of dyes to school Art and Design and Textile Departments. They also provide kits of dyes and fabrics for similar demonstrations.
- The SHIPS Resource Center at the University of Minnesota provides a cross-curricular unit called ‘A Lesson to Dye For’, which also highlights investigative skills. The level of chemistry is not high, but the range of ideas is very wide, and teachers may find some useful ideas for follow-up work.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet