Compare the chlorine content and concentration of sodium hypochlorite in different bleaches in this class practical
In this experiment, students add excess hydrogen peroxide to measured samples of household bleach, collecting and measuring the volume of oxygen produced. They then use this data to compare the chlorine content of different bleaches, and calculate the concentration of sodium chlorate(I) (sodium hypochlorite).
This experiment can be done as a demonstration as well as a class practical (with suitable students). Younger students can compare the relative concentration or ‘value’ of different bleaches. Intermediate students could determine concentration and revise redox reactions and half-equations. Advanced students could make use of oxidation numbers to balance equations, consider electrode potentials for the reactions, and also meet hydrogen peroxide behaving as a reducing agent.
A few commercial bleaches in their containers, with prices, can be placed on a suitable tray, each with a 10 cm3 syringe and 250 cm3 beaker – both labelled – into which small samples of the bleach can be placed. Students can measure 5 cm3 of each bleach into their side-arm flask for each experiment. Small samples of the hydrogen peroxide solution could be collected in a 100 cm3 beaker.
Students should have no access to acid solutions. Bleaches liberate toxic chlorine gas on contact with acids.
Depending on the apparatus used and the number of bleaches investigated, the practical could be completed in 15–45 minutes.
Equipment
Apparatus
- Eye protection
- Conical side-arm flask, 250 cm3
- Bung, with single hole to fit 10 cm3 syringe nozzle
- Plastic syringe, to deliver 10 cm3 (see note 5 below)
- Plastic syringe, to deliver 5 cm3 (see note 6)
- Delivery tube (see diagram below)
- Rubber tubing, short length
- Water trough or washing-up bowl
- Measuring cylinder, 100 cm3
- Measuring cylinder, 25 cm3
- Beaker, 100 cm3
- Clamp and stand, x2
Chemicals
- Deionised or distilled water, 25 cm3
- A variety of household bleach solutions (IRRITANT) (see note 3 below)
- Hydrogen peroxide, ‘20 volume’ solution (IRRITANT at this concentration), 10 cm3 per bleach solution
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Household bleach solutions (containing sodium chlorate(I) / sodium hypochlorite) (unlikely to be CORROSIVE but may be IRRITANT) – see CLEAPSS Hazcard HC089. Commercial household bleaches usually contain about 5% sodium chlorate(I). Some bleaches also contain detergents and thickening agents, which may cause excessive frothing in this experiment. Note that nowadays some commercially available bleaches do not contain any chlorine and are based on peroxy-compounds. They should not be used here.
- Hydrogen peroxide solution, H2 O2 (aq), (IRRITANT at concentration used) – see CLEAPSS Hazcard HC050 and CLEAPSS Recipe Book RB045.
- Plastic syringes are used to measure and deliver a known volume of hydrogen peroxide solution, and their nozzles should fit tightly into the hole in the flask bung.
- Plastic syringes can be used to measure 5 cm3 of bleach solution, but volumetric pipettes with safety fillers could be used instead.
Procedure
Note
Splashes of bleach and hydrogen peroxide should be washed off immediately with plenty of water.
- Use a plastic syringe to measure out 5 cm3 of the first bleach into the flask. If a ‘thick’ bleach is used, add approximately 25 cm3 deionised water. Swirl to ensure complete mixing.
- Discuss the fact that this dilution does not change the amount of bleach put into the flask, but does enable proper mixing to take place and ensure the reaction has goes to completion.
- Half fill the trough with water. Submerge the 100 cm3 measuring cylinder and fill it with water, invert under water and clamp it in position.
- Attach the delivery tube to the side arm flask and arrange the rest of the apparatus as shown in the diagram below.
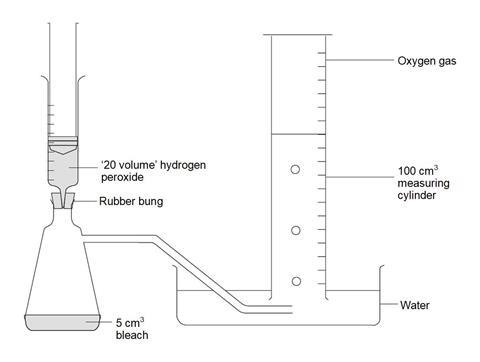
- Measure 10 cm3 hydrogen peroxide solution into a clean plastic syringe, attach it to the bung and gently empty the contents into the flask. Leave the syringe in place.
- Carefully swirl the contents of the conical flask and collect the gas liberated.
- Continue until no further reaction is seen. Measure and record the final volume.
- Carefully disconnect the delivery tube from the flask.
- Discard the solution into the sink. Flush away with plenty of water. Rinse the flask thoroughly. Ensure any splashes of bleach are washed off skin immediately and swabbed off benches.
- If time allows, repeat the experiment with the same bleach to obtain three results and take an average.
- Repeat the experiment with different bleaches.
Teaching notes
Here hydrogen peroxide behaves as reducing agent with another more powerful oxidising agent. (More advanced students may expect it to behave as an oxidising agent.)
Half-equation:
H2O2→ O2 + 2H+ + 2e-
Chlorate(I) ions are an oxidising agent.
Half-equation:
OCl– + 2e– → O2– + Cl–
Overall equation showing the change of oxidation state of chlorine:
H2O2(aq) + NaOCl(aq) → H2O(l) + NaCl(aq) + O2(g)
The maximum volume of gas recorded in the measuring cylinder should have 10 cm3 deducted from it to compensate for the injection of 10 cm3 hydrogen peroxide solution into the flask.
Direct comparison of volume of oxygen collected in the measuring cylinder compares the effectiveness of the bleaches for younger students.
’Value’ can be compared by dividing the volume of oxygen liberated by the cost of 5 cm3 of bleach. This extra step is well within the grasp of most introductory students
Calculation of concentration (in g NaOCl per dm3) for more advanced students:
- (V–10) cm3 is the volume of oxygen liberated.
- (V–10)/24000 is the number of moles of oxygen gas.
- (V–10)/24000 is the number of moles of NaOCl in 5 cm3 bleach (see equations above).
- (V–10)/24000 x 1000/5 is the number of moles of bleach in a 1 dm3 bottle of bleach.
- ((V–10)/24000 x 1000/5) x 74.5 is the mass of NaOCl in 1 dm3 of bleach solution.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet