Use this demonstration to illustrate catalysis of the oxidation of potassium sodium tartrate by hydrogen peroxide
Catalysts provide an alternative route for reactions to proceed – they are involved in the progress of the reaction. In this exciting and spectacular demonstration, students observe as cobalt(II) chloride is added to a mixture of potassium sodium tartrate and hydrogen peroxide, catalysing the oxidation of tartrate ions and producing a vivid colour change.
The experiment lasts about 15 minutes, or longer if explaining the chemistry to an A-level audience. It takes about 30 minutes to set up.
Equipment
Apparatus
- Eye protection
- Beaker, 250 cm3
- Measuring cylinder, 50 cm3
- Measuring cylinder, 10 cm3
- Tripod
- Gauze
- Bunsen burner
- Thermometer, –10–110 °C
- Access to balance (1 d.p.)
Chemicals
- Potassium sodium tartrate (Rochelle salt), 5 g
- Hydrogen peroxide, 20 ‘volume’ (IRRITANT), 20 cm3
- Cobalt(ll) chloride solution, 4% (TOXIC, DANGEROUS FOR THE ENVIRONMENT), 5 cm3
- Distilled or deionised water, 60 cm3
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Potassium sodium tartrate (Rochelle salt), KNaC4H4O6.4H2O(s) – see CLEAPSS Hazcard HC036c.
- Hydrogen peroxide solution, H2O2(aq), (IRRITANT) – see CLEAPSS Hazcard HC050 and CLEAPSS Recipe Book. The 20 ‘vol’ solution is best made by diluting fresh 100 ‘vol’ hydrogen peroxide solution (HARMFUL, wear goggles and consider wearing gloves) for this demonstration.
- Cobalt(ll) chloride solution, CoCl2(aq), (TOXIC) – see CLEAPSS Hazcard HC025 and CLEAPSS Recipe Book. Dissolve 0.2 g of cobalt(II) chloride-6-water (TOXIC, DANGEROUS FOR THE ENVIRONMENT) in 5 cm3 of distilled or deionised water.
Procedure
- Weigh 5 g of the potassium sodium tartrate into a 250 cm3 beaker. Add 60 cm3 of distilled water and stir to dissolve the solid.
- Add 20 cm3 of 20 volume hydrogen peroxide solution to the solution in the beaker. Note any signs of reaction.
- Put the beaker on the tripod and gauze and heat the mixture in the beaker to about 70 °C. Note any signs of reaction.
- Add 5 cm3 of cobalt(ll) chloride solution to the mixture in the beaker. Take care to avoid skin contact. Note any colour changes and gas produced.
Teaching notes
It is worthwhile trying this experiment before carrying out in class, because it is important to note the colour changes that occur when carbon dioxide gas is evolved.
This is an impressive demonstration of how a catalyst is involved in the progress of a reaction. Students can add another 10 cm3 of the hydrogen peroxide solution and if there is any potassium sodium tartrate remaining they will see a similar reaction.
The reaction is an oxidation of the tartrate ion (proper name is 2,3-dihydroxybutandioate ion) to carbon dioxide gas and the methanoate ion. Hydrogen peroxide oxidises the tartrate ion very slowly if there is no catalyst, even at elevated temperatures.
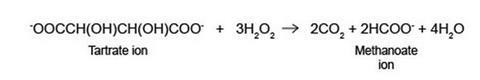
Cobalt(ll) ions are pink. The hydrogen peroxide initially oxidises the cobalt(II), Co2+, to cobalt(lll), Co3+, which is green. The cobalt(III) bonds to the tartrate ion, allowing the oxidation to take place. The Co3+ is then reduced back to Co2+ and the pink colour returns.
The cobalt catalyst provides an alternative route for the reaction to occur. This alternative route has a lower activation energy and the reaction proceeds much more quickly.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry. This collection of over 200 practical activities demonstrates a wide range of chemical concepts and processes. Each activity contains comprehensive information for teachers and technicians, including full technical notes and step-by-step procedures. Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet