Use the experiment to introduce Le Chatelier’s principle, or encourage students to apply the principle to make predictions
For this activity, the demonstrator fills a gas syringe with an equilibrium mixture of brown nitrogen dioxide and colourless dinitrogen tetroxide. The effect of pressure on the equilibrium is shown to be in accordance with Le Chatelier’s principle by compressing the mixture and observing the change in colour intensity.
Similarly, the effect of temperature is demonstrated by heating or cooling the mixture and observing the change in colour, or the change in volume of the mixture compared with that of a similar volume of air.
The demonstration can be used to introduce Le Chatelier’s principle or to ask students to apply it in predicting the changes expected when pressure and temperature are changed.
Preparation must be carried out in a fume cupboard. The filled syringes may be brought out onto a lecture bench in a well ventilated room for better visibility provided they are sealed and do not leak when put under pressure. The nitrogen oxides involved are very toxic and students with breathing problems should avoid inhaling them as they could trigger an asthma attack.
A white background will greatly enhance the visibility of the colour changes.
The time for carrying out the demonstration, including preparation of the dinitrogen tetroxide, should be about 30–40 minutes. More time will be needed for the quantitative extension option.
Equipment
Apparatus
- Eye protection (for the demonstrator)
- Access to a fume cupboard
- Clear glass gas syringes, 100 cm3, x2 or x3 (note 3)
- Rubber septum caps to fit syringe tips, x2 or x3 (note 4)
- Plastic tubing, short lengths (optional; note 5)
- Screw clips (Hoffmann) for rubber tubing, x2 or x3 (optional; note 5)
- Boiling tube with a sidearm
- Hard glass (borosilicate) boiling tube
- One-holed rubber stoppers to fit the boiling tubes, x2
- Glass tubing, short lengths, x2 (to fit stoppers and bent as shown in the diagram below)
- Spring clip (Mohr) for rubber tubing (optional)
- Three-way stopcock or glass (or plastic) T-piece
- Beaker, 400 cm3
- Beakers, 2 dm3, x2
- Thermometer (-10–110 °C)
- Bunsen burner
- Tripod
- Gauze
- Bosses, clamps and stands as required
Chemicals
- Lead(II) nitrate(V) (TOXIC, DANGEROUS FOR THE ENVIRONMENT), 10–20 g (note 6)
- Crushed ice, about 400 cm3
- Common salt (sodium chloride) or crushed rock salt, about 100 g
- Light lubricating oil (such as WD-40), a few drops
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection and work in a fume cupboard.
- Two or three gas syringes is ideal, but the experiment can be done with one.
- The syringes should all be airtight under pressure. If not, lubricate the plunger with a few drops of light mineral oil, such as sewing machine oil or WD-40.
- Instead of using septum caps to seal the syringes, a short length of plastic tubing carrying a screw clip can be used to connect the syringe to the sidearm tube. The tubing must fit the syringe tightly enough not to be dislodged when the gas is put under pressure. Soften it by warming it in hot water before attaching it to the syringe. However, it must not fit the sidearm tube so tightly that it cannot easily be disconnected from it when the syringe has been filled.
- Lead(II) nitrate(V), Pb(NO3)2 (s), and lead(II) oxide, PbO(s), (both TOXIC, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcards HC057a and HC056.
The lead(II) nitrate should be thoroughly dried by heating overnight in an oven set to around 100 °C. Store in a desiccator after heating unless used immediately.
The equation for the decomposition of lead nitrate is Pb(NO3)2 (s) → PbO(s) + 2NO2 (g) + 1/2 O2 (g).
1 g of lead(II) nitrate(V) should therefore produce about 150 cm3 of NO2 gas at room temperature and pressure. Adjust the mass of lead nitrate accordingly (but use at least 5 g) depending on the number of syringes of gas required, bearing in mind the number of times they are flushed (see below) before finally filling.
The lead(II) oxide residue will soften the glass of the test tube and fuse with it. This combined residue should be collected and labelled as hazardous waste, for eventual collection by a licensed contractor – see CLEAPSS Hazcard HC056. - Nitrogen dioxide, NO2 (g), and dinitrogen tetroxide, N2 O4, (both VERY TOXIC) – see CLEAPSS Hazcard HC068B.
- Sodium chloride, NaCl(s) – see CLEAPSS Hazcard HC047b.
Procedure
Before the demonstration
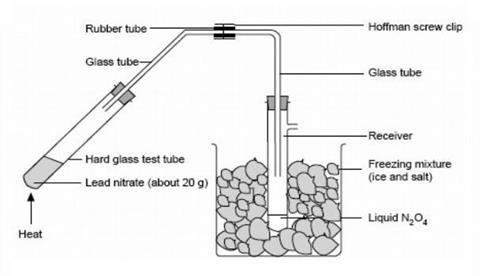
- In a fume cupboard, set up the apparatus for collection of dinitrogen tetroxide shown in the diagram below. Stand the smaller beaker on a gauze on a tripod and clamp both of the test tubes near the top. Keep the length of the rubber connecting tube as short as possible as nitrogen dioxide attacks rubber.
- It is vital that a syringe does not leak under pressure. Test if a syringe is airtight by sealing it using a rubber septum cap, with about 60 cm3 of air in the syringe. Hold the syringe vertically with septum cap resting on a firm support, such as the bench surface. Push in the plunger to compress the gas as far as comfortably possible, hold for a few seconds, then release the pressure. The plunger should return to its original position if the syringe is airtight. If not, check the septum cap, or replace the syringe.
- Mix the salt with the crushed ice to produce a freezing mixture and place it in the beaker.
The demonstration
- Working in a fume cupboard, heat the lead nitrate gently to decompose it to nitrogen dioxide (and oxygen). Heating too vigorously may decompose the nitrogen dioxide into nitrogen monoxide. As the gas meets the cold wall of the sidearm test tube, it condenses as liquid dinitrogen tetroxide, which may appear greenish in colour due to dissolved water if the lead nitrate was not absolutely dry. The oxygen is lost to the atmosphere.
- When about 2 cm3 of liquid has been collected, stop heating, tighten the screw clip and disconnect the tube containing the lead nitrate residue, leaving the screw clip sealing the tube connected to the receiver. The liquefied dinitrogen tetroxide can be kept for some time in the freezing mixture, so that this part of the experiment could be done before the demonstration if desired.
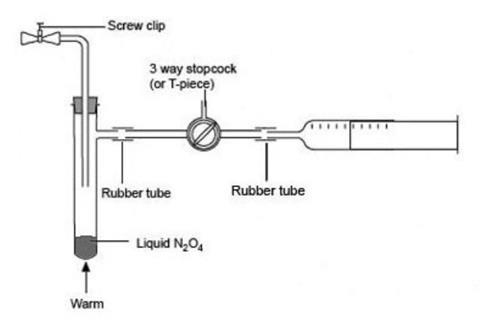
- Connect the sidearm of the tube containing the dinitrogen tetroxide to a syringe nozzle via the three-way stopcock or T-piece and short lengths of rubber tubing (see diagram below). If a T-piece is used, the third outlet can be opened or closed using a gloved finger (for preference, use nitrile gloves) or a short length of rubber tubing fitted with a spring clip.
- Set the stopcock or T-piece to open the connection between the syringe and the sidearm tube and partly fill the syringe with the brown gas mixture by removing the ice bath and gently warming the sidearm tube in your hand or a beaker of warm water. As the almost colourless dinitrogen tetroxide evaporates, it decomposes to form some brown nitrogen dioxide.
- Flush the first filling of gas out of the syringe into the fume cupboard using the three-way tap or the T-piece, and repeat the filling and flushing cycle two or three more times to ensure that there is no air in the system. Finally fill the syringe to the 50–60 cm3 mark, release it from the connecting rubber tubing and quickly seal it with a septum cap. Leave the tap or T-piece open to allow any excess gas to flush out into the fume cupboard (see diagram below).
The effect of pressure on the equilibrium
- In good view of the class and against a white background, hold the syringe with the septum cap resting on a hard surface. Compress the gas mixture rapidly by pressing in the plunger of the syringe as far as it will comfortably go and hold it there for a few seconds. The colour of the gas mixture will initially become darker as the concentration of the gases increases with the decrease in volume. Within a few seconds, however, it will become paler as the equilibrium responds to the increased pressure and brown nitrogen dioxide is converted into colourless dinitrogen tetroxide.
- Pull the plunger out to its starting position. Note the colour changes as the pressure is decreased. They should be the reverse of the above.
The effect of temperature on the equilibrium
- Fill one large beaker about two-thirds full with ice-cold water and another with hot water at about 60–70 °C.
- Fill one, two or three syringes with 50–60 cm3 of the brown gas mixture as above and seal them with septum caps. If only one syringe is used, let the class observe the colour of its contents against a white background, then place it in the beaker of hot water. The gas will expand and become darker as the equilibrium adjusts to the higher temperature by converting dinitrogen tetroxide to nitrogen dioxide.
- Transfer the syringe (or use another syringe full of gas) into the cold water. The gas will contract but become lighter in colour as the equilibrium readjusts to the decrease in temperature. If three syringes of gas are used, one can be kept at room temperature for comparison.
Teaching notes
The gaseous equilibrium studied here is: N2O4(g) ⇌ 2NO2(g) ΔHo = +58 kJ mol–1
Le Chatelier’s principle predicts that the equilibrium will move to the right with an increase in temperature as the forward reaction is endothermic. The brown colour intensifies. The equilibrium constant, Kp, has a value of 48 atm at 400 K (127 °C), and the equilibrium will lie almost completely over to the right at 140 °C.
Increasing the pressure moves the equilibrium to the left as this decreases the number of moles of gas present in the mixture. The brown colour becomes paler.
Remind students that the value of Kp will increase with increase in temperature, whereas it will not be affected by changes in pressure despite a shift in the position of equilibrium.
A quantitative extension: comparison with ideal gas behaviour (optional)
By recording the volume of the gas mixture at intervals as it is warmed up, a plot of volume vs temperature can be obtained – see graph below. This can be compared with volume readings obtained from a syringe containing a similar volume of air.
- Clamp two syringes, one containing the mixture of nitrogen oxides and one containing a similar volume of air (about 50 cm3), vertically in a 2 dm3 beaker of water on a tripod and gauze, so that they are immersed up to the 100 cm3 mark.
- Note the volume readings of both syringes, which should be as close as possible, and the temperature of the water. Heat the water gently with a Bunsen burner and record the temperature and the volume of gases every 10 °C or so. Before taking each reading, remove the Bunsen burner and stir the water for a couple of minutes to ensure that the temperature of the gases is the same as that of the water. Twist the plungers of the syringes before taking each reading, to ensure that they are not sticking.
- Continue taking readings until the temperature is between 70 to 80 °C. Plot graphs of volume against temperature for both gases on the same axes. The nitrogen dioxide/nitrogen tetroxide mixture will expand more than air (see diagram below) as the equilibrium responds to the increase in temperature by producing more nitrogen dioxide. If there is time take further readings as the water cools, to check for leaks.
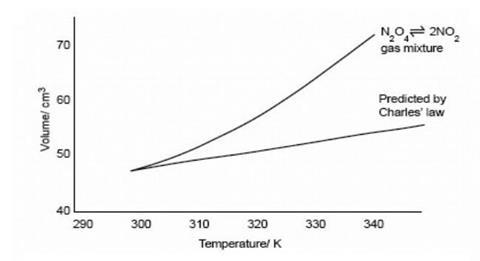
This experiment can be done without a second syringe of air if necessary. The predicted volume of an ideal gas can be worked out using Charles’ Law for each temperature reading and compared with the observed one (see graph above). For example, if the volume of the gas mixture is 50 cm3 at 25 °C (298 K), the predicted volume of an ideal gas at 50 °C (323 K) would be 50 x 323/298 = 54.2 cm3. Students could watch the demonstration and plot the points as the readings are taken. Alternatively they could do the Charles’ Law calculations as the equilibrium mixture is warmed up.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry
Equilibrium and Le Chatelier’s principle
- 1
- 2
- 3
- 4
 Currently reading
Currently readingThe effect of pressure and temperature on equilibrium





















No comments yet