Try this demonstration to determine the formula of water through the reaction of copper(II) oxide with hydrogen
In this experiment, students observe as a known mass of heated copper(II) oxide is reduced in a stream of hydrogen gas. The water formed by this reaction is then absorbed by sulfuric acid or anhydrous calcium chloride granules.
The loss in mass of the oxide is equal to the mass of the oxygen in the water, while the gain in mass of the whole apparatus is equal to the mass of the hydrogen in the water. From these results, students can work out the percentage by mass composition of water and deduce its formula.
This demonstration extends the investigation of the volumes of hydrogen and oxygen that combine (see this experiment exploring the combustion of hydrogen in air) to a more quantitative level involving reacting masses. It requires careful rehearsal if results approaching the expected values for the composition of water are to be obtained.
The ratio of the reacting masses can be used to deduce a relative atomic mass for oxygen, based on hydrogen. The reacting masses can also be used to deduce the formula for water and the balanced equation for its formation. This could be extended to a treatment using the mole concept with suitable groups.
The time for carrying out the demonstration should be about 30–40 minutes.
Equipment
Apparatus
- Eye protection for demonstrator
- Safety screens
- Side-arm test tubes, 140 x 22 mm, x2
- Test tubes, x2
- One-hole bung, to fit side-arm tubes, x2
- Right-angled glass delivery tubes, x2
- Short length of glass tubing, to fit bung
- Combustion tube, about 15 cm long
- Drying tube
- One-holed bung, to fit drying tube
- Right-angled glass tube with jet
- Short lengths of rubber tubing
- Glass or ceramic wool
- Dropping pipette teats, to seal glass tubes, x2
- Bunsen burner
- Access to a balance
- Boss, clamp and stand
Chemicals
- Copper(II) oxide, wire form, (HARMFUL, DANGEROUS FOR THE ENVIRONMENT), 25–30 g
- Concentrated sulfuric acid (CORROSIVE), about 20 cm3
- Anhydrous calcium chloride granules (IRRITANT), enough to fill drying tube
- Access to a supply of hydrogen (EXTREMELY FLAMMABLE), cylinder or chemical generator – see these standard techniques for generating, collecting and testing gases
Health, safety and technical notes
- Read our standard health and safety guidance.
- Wear eye protection throughout.
- Copper(II) oxide, CuO(s), purchased in wire form (HARMFUL, DANGEROUS FOR THE ENVIRONMENT) – see CLEAPSS Hazcard HC026. The copper(II) oxide should be thoroughly dried by heating at 300–400 °C for a few minutes and stored, when cool, in a desiccator.
- Concentrated sulfuric acid (CORROSIVE) – see CLEAPSS Hazcard HC098a.
- Anhydrous calcium chloride, CaCl2 (s), (IRRITANT) – see CLEAPSS Hazcard HC019A. The anhydrous calcium chloride must be freshly opened or thoroughly dried by heating in a Bunsen flame and stored, when cool, in a desiccator.
- Hydrogen, H2 (g), (EXTREMELY FLAMMABLE) – see CLEAPSS Hazcard HC048. Using hydrogen from a gas cylinder will be much more efficient in flushing the air out of the apparatus, which has quite a considerable total volume.
Procedure
- Place 25 – 30 g of dry wire-form copper(II) oxide in the combustion tube and secure in it place with tufts of glass or ceramic wool. Fit the bung and glass tube. Weigh the tube and its contents.
- Place a few cm3 of concentrated sulfuric acid in each of the side-arm test tubes and fit the bungs carrying the delivery tubes. Make sure that the ends of the tubes are below the level of the acid. Connect one of the side-arm tubes to the exit end of combustion tube, as shown in the figure below. Seal the ends of this assembly with pipette teats and weigh it.
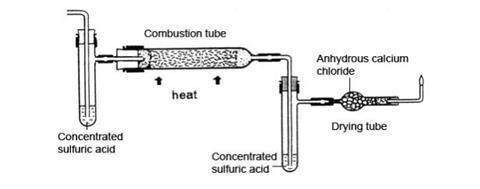
- Attach the second side-arm tube to the end of the combustion where the hydrogen gas enters, to capture any moisture in the gas. Fill the drying tube with anhydrous calcium chloride granules using a plug of glass or ceramic wool to hold them in position. Add the tube carrying the jet and join the drying tube to the rest of the apparatus, as shown in the diagram above. Seal the apparatus with the teats again.
- Clamp the apparatus in position, remove the teats and connect it to the hydrogen supply, set beforehand to deliver a steady stream of gas.
- It is most important to test the gas passing through the apparatus before heating is begun, As the apparatus has a considable volume, it will take time to flush out all the air. Take samples of the gas issuing from the jet using an inverted test tube. Attempt to ignite the hydrogen by passing the mouth of the test tube through a Bunsen flame situated at a safe distance from the apparatus. If it ignites with a pop then there is still air in the mixture. If it merely ignites and burns smoothly, this indicates that the gas is almost pure hydrogen.It should then be possible to use the burning gas to light the hydrogen jet.
- Now heat the contents of the combustion tube with a medium-sized Bunsen flame until all the copper oxide has been reduced to copper. It will change colour from black to a bright, pinkish-coloured solid. Water will condense inside the combustion tube. It is important to reduce all the oxide and to continue heating until no more moisture comes out of the combustion tube and is absorbed by the sulphuric acid or the drying tube.
- Continue the flow of gas until the combustion tube has cooled down. Stop the gas flow and disconnect it from the apparatus. Remove the first side-arm tube and the drying tube and seal the combustion tube + second side-arm tube assembly using the two teats. Weigh it as a whole before removing the combustion tube and weighing it separately.
Teaching notes
The redox reaction is simply: CuO(s) + H2(g) → Cu(s) + H2O(l)
Use the masses of the combustion tube before and after heating to work out the mass of oxygen lost by the copper(II) oxide and now present in the water formed.
Use the mass of the whole apparatus before and after heating to work out the mass of water formed and hence the mass of hydrogen in it by subtracting the mass of oxygen. As the oxygen is only transferred from the copper oxide to form water inside the apparatus, any gain in mass is due to hydrogen combined with the oxygen to form water.
The ratio of the two masses enables the mass of oxygen combining with 1 g of hydrogen to be calculated. It should be close to 8 g if all has gone well. Point out that this would give oxygen a relative mass of 8 on a scale where H = 1, if the formula of water was HO.
However, as the relative mass of an oxygen atom is 16, this mass ratio is in accordance with a formula for water of H2O.
Additional information
This is a resource from the Practical Chemistry project, developed by the Nuffield Foundation and the Royal Society of Chemistry.
Practical Chemistry activities accompany Practical Physics and Practical Biology.
© Nuffield Foundation and the Royal Society of Chemistry


















No comments yet