When teaching the reactivity series, aluminium’s protective oxide layer can make it difficult for students to see its true reactivity in the context of metals reacting with aqueous solutions. A demonstration involving mercury(II) chloride is described in Classic chemistry demonstrations and can be found on the Practical Chemistry website. That experiment shows how removing the protective layer causes the aluminium to react with air. However, this demonstration shows its reaction in solutions that students are likely to have already met. With a little practice, it has the added wow factor of burning with an eerie green flame that dances in the vessel as hydrogen is evolved.
Kit
The dancing flame effect can be achieved in a variety of different ways. These instructions describe one reproducible method.
- 2 × 250 cm3 conical flasks. Aim for a generous neck width (I use 34 mm internal diameter)
- Aluminium kitchen foil, 2 sheets ~20 cm × 8 cm
- ~3.5 g CuCl2•2H2O (harmful if swallowed, solid may irritate the eyes and skin, danger to the environment)
- ~3.5 g CuSO4•5H2O (harmful, solid may irritate the eyes and skin, water added to the anhydrous solid (sulfate VI) produces heat danger to the environment)
- 50 cm3 1M HCl (low hazard)
- 50 cm3 0.5M H2SO4 (irritant)
Preparation
Dissolve the copper(II) sulfate in the sulfuric acid and the copper(II) chloride in the hydrochloric acid and set the two solutions aside. Take a piece of aluminium foil approximately the width of the base of the conical flask and approximately 20 cm long. Roll it loosely just enough to be able to fit through the neck of the flask – use a splint or spatula to gently push it home so it lies on its side on the base of the flask. Repeat with the other conical flask. Have a source of ignition nearby with some splints.
In front of the audience

When meeting the reactivity series for the first time, it is likely that students will have experimented with adding strips of metals to solutions of metal compounds, including copper sulfate. Show the students the solution of copper sulfate and explain that it also contains sulfuric acid. Invite predictions of what may occur. Students may predict that hydrogen will be produced from the reaction with acid and if they are already familiar with displacement reactions they may predict that the aluminium will dissolve and copper will be formed. Few are likely to predict changes to the colour of the solution without prompting.
When the mixture is poured on the aluminium foil, nothing appears to happen. It is at this point that you may choose to tell students about the protective layer of aluminium oxide, which is stable enough in dilute acid that it prevents us from seeing aluminium’s true reactivity. In the other solution, the chloride ions can disrupt this layer and reveal how reactive it can be. You may wish to point out that the effective concentrations of the two copper compounds are the same, as are the two acids (the formula masses of the two hydrated compounds are similar enough that this is a fair approximation).
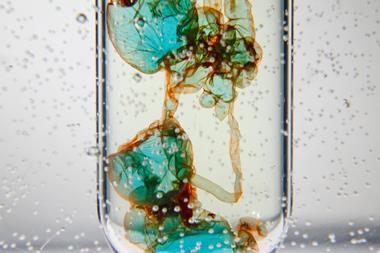
Add the copper chloride-hydrochloric acid mixture to the foil in the second flask and immediately light a splint. After a few seconds, the mixture will begin to react vigorously and produce hydrogen gas. Hold the lit splint by the opening of the flask and the gas will ignite. If you have timed it right, the flame will sink back into the flask and dance inside above the reaction with an eerie green colour from the copper. As the reaction slows, the flame will extinguish itself. Swirl the flask to reinvigorate the reaction before attempting to relight.
The most common problem with this demonstration is finding that you can only get one ‘squeaky pop’ style ignition of the gas or that the flame retreats to the base of the flask and self-extinguishes. Further attempts to light the hydrogen in the flask result in you dropping the splint down into the vessel, after which it promptly self-extinguishes as there is little air present. The reaction is proceeding too slowly to continue feeding hydrogen at the appropriate rate. Repeat the reaction with a little more copper chloride in the mixture.
Teaching goal
The primary objective here is to show students how reactive aluminium is. The aluminium oxide layer protects the metal beneath from further reaction with air, water or acid. But chloride ions can ligate aluminium ions at the metal–oxide interface and break down the protective layer, allowing the reaction to proceed.
The following reactions are by necessity a ‘good enough’ explanation for this level as a range of aluminium species are involved. These are consistent with the level at which the demonstration is aimed, and with reactions that students may come across later in their school careers.
2Al(s) + 3CuCl2(aq) → 2AlCl3(aq) + 3Cu(s)
2Al(s) + 6HCl(aq) → 2AlCl3(aq) + 3H2(g)
Disposal
All solutions can be washed down the sink with plenty of water. Pour the flask contents through a sieve to catch any large pieces of unreacted foil.
Further reading
- The real reactivity of aluminium in either Practical Chemistry or T Lister, Classic Chemistry Demonstrations, p39. Royal Society of Chemistry, 1996
- A demonstration from Periodic Table of Videos using an aluminium cake casing is well worth watching
- The reaction of aluminium with copper salts
Safety
Wear eye protection.

















1 Reader's comment