Can France claim the first periodic table? Probably not, but a French Geology Professor made a significant advance towards it, even though at the time few people were aware of it.
Alexandre Béguyer de Chancourtois was a geologist, but this was at a time when scientists specialised much less than they do today. His principal contribution to chemistry was the 'vis tellurique' (telluric screw), a three-dimensional arrangement of the elements constituting an early form of the periodic classification, published in 1862.
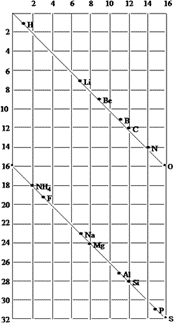
The telluric screw plotted the atomic weights of the elements on the outside of a cylinder, so that one complete turn corresponded to an atomic weight increase of 16. As the diagram shows, this arrangement means that certain elements with similar properties appear in a vertical line. Although the telluric screw did not correctly display all the trends that were known at the time, de Chancourtois was the first to use a periodic arrangement of all of the known elements, showing that similar elements appear at periodic atom weights.
The vis tellurique from De Chancourtois’s original publication (right) and a copy drawn out with modern symbols (left). |
|
|

John Newlands was British; his father was a Scottish Presbyterian minister. He was educated by his father at home, and then studied for a year (1856) at the Royal College of Chemistry, which is now part of Imperial College London. Later he worked at an agricultural college trying to find patterns of behaviour in organic chemistry. However, he is remembered for his search for a pattern in inorganic chemistry.
Just four years before Mendeleev announced his periodic table, Newlands noticed that there were similarities between elements with atomic weights that differed by seven. He called this The Law of Octaves, drawing a comparison with the octaves of music. The noble gases (Helium, Neon, Argon etc.) were not discovered until much later, which explains why there was a periodicity of 7 and not 8 in Newlands table. Newlands did not leave any gaps for undiscovered elements in his table, and sometimes had to cram two elements into one box in order to keep the pattern. Because of this, the Chemical Society refused to publish his paper, with one Professor Foster saying he might have equally well listed the elements alphabetically.
Even when Mendeleev had published his table, and Newlands claimed to have discovered it first, the Chemical Society would not back him up. In 1884 he was asked to give a lecture of the Periodic Law by the Society, which went some way towards making amends. Finally, in 1998 the Royal Society of Chemistry oversaw the placing a blue commemorative plaque on the wall of his birthplace, recognising his discovery at last.
| The blue commemorative plaque placed at Newlands’ birthplace, declaring him the “discoverer of the Periodic Law for the chemical elements”. |

Meyer trained at Heidelberg University under Bunsen and Kirchhoff, as did Mendeleev. So the two scientists would certainly have known each other although neither was aware of all the work done by the other. Meyer's roots, however, were firmly in Germany. Meyer was just four years older than Mendeleev, and produced several Periodic Tables between 1864-1870.
His first table contained just 28 elements, organised by their valency (how many other atoms they can combine with). These elements were almost entirely main group elements, but in 1868 he incorporated the transition metals in a much more developed table. This 1868 table listed the elements in order of atomic weight, with elements with the same valency arranged in vertical lines, strikingly similar to Mendeleev’s table. Unfortunately for Meyer, his work wasn’t published until 1870, a year after Mendeleev’s periodic table had been published. Even after 1870, Meyer and Mendeleev were still unaware of each other’s work, although Meyer later admitted that Mendeleev had published his version first.
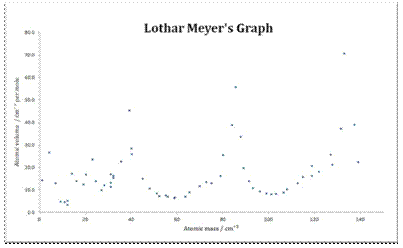
Meyer did contribute to the development of the periodic table in another way though. He was the first person to recognise the periodic trends in the properties of elements, and the graph shows the pattern he saw in the atomic volume of an element plotted against its atomic weight.
| A modern version of Meyer’s graph demonstrating the periodic trends in the atomic volume of the elements, plotted against atomic weight. |

As we have seen, Mendeleev was not the first to attempt to find order within the elements, but it is his attempt that was so successful that it now forms the basis of the modern periodic table.
Mendeleev did not have the easiest of starts in life. He was born at Tobolsk in 1834, the youngest child of a large Siberian family. His father died while he was young, and so his mother moved the family 1500 km to St. Petersburg, where she managed to get Dmitri into a “good school“, recognising his potential. In his adult life he was a brilliant scientist, rising quickly in academic circles. He wrote a textbook, Chemical Principles, because he couldn’t find an adequate Russian book.
Mendeleev discovered the periodic table (or Periodic System, as he called it) while attempting to organise the elements in February of 1869. He did so by writing the properties of the elements on pieces of card and arranging and rearranging them until he realised that, by putting them in order of increasing atomic weight, certain types of element regularly occurred. For example, a reactive non-metal was directly followed by a very reactive light metal and then a less reactive light metal. Initially, the table had similar elements in horizontal rows, but he soon changed them to fit in vertical columns, as we see today.
Not only did Mendeleev arrange the elements in the correct way, but if an element appeared to be in the wrong place due to its atomic weight, he moved it to where it fitted with the pattern he had discovered. For example, iodine and tellurium should be the other way around, based on atomic weights, but Mendeleev saw that iodine was very similar to the rest of the halogens (fluorine, chlorine, bromine), and tellurium similar to the group 6 elements (oxygen, sulphur, selenium), so he swapped them over.
The real genius of Mendeleev’s achievement was to leave gaps for undiscovered elements. He even predicted the properties of five of these elements and their compounds. And over the next 15 years, three of these elements were discovered and Mendeleev’s predictions shown to be incredibly accurate. The table below shows the example of Gallium, which Mendeleev called eka-aluminium, because it was the element after aluminium. Scandium and Germanium were the other two elements discovered by 1886, and helped to cement the reputation of Mendeleev’s periodic table.
The final triumph of Mendeleev’s work was slightly unexpected. The discovery of the noble gases during the 1890s by William Ramsay initially seemed to contradict Mendeleev’s work, until he realised that actually they were further proof of his system, fitting in as the final group on his table. This gave the table the periodicity of 8 which we know, rather than 7 as it had previously been. Mendeleev never received a Nobel Prize for his work, but element 101 was named Mendelevium after him, an even rarer distinction.
Eka-aluminium (Ea) | Gallium (Ga) | |
Atomic weight | About 68 | 69.72 |
Density of solid | 6.0 g/cm³ | 5.9 g/cm³ |
Melting point | Low | 29.78°C |
Valency | 3 | 3 |
Method of discovery | Probably from its spectrum | Spectroscopically |
Oxide | Formula Ea2O3, density 5.5 g/cm3. Soluble in both acids and alkalis | Formula Ga2O3, density 5.88 g/cm3. Soluble in both acids and alkalis |
A comparison of Mendeleev’s predicted “Eka-aluminium” and Gallium, discovered by Paul Emile Lecoq in 1875
| A commemorative stamp showing Mendeleev and some of his original notes about the Periodic Table |

It wasn’t until 1913, six years after Mendeleev’s death that the final piece of the puzzle fell into place. The periodic table was arranged by atomic mass, and this nearly always gives the same order as the atomic number. However, there were some exceptions (like iodine and tellurium, see above), which didn’t work. Mendeleev had seen that they needed to be swapped around, but it was Moseley that finally determined why.
He fired the newly-developed X-ray gun at samples of the elements, and measured the wavelength of X-rays given. He used this to calculate the frequency and found that when the square root of this frequency was plotted against atomic number, the graph showed a perfect straight line. He’d found a way to actually measure atomic number. When the First World War broke out, Moseley turned down a position as a professor at Oxford and became an officer in the Royal Engineers. He was killed by a sniper in Turkey in August 15, and many people think that Britain lost a future Nobel prize winner.
Within 10 years of his work, the structure of the atom had been determined through the work of many prominent scientists of the day, and this explained further why Moseley’s X-rays corresponded so well with atomic number. The idea behind the explanation is that when an electron falls from a higher energy level to a lower one, the energy is released as electromagnetic waves, in this case X-rays. The amount of energy that is given out depends on how strongly the electrons are attracted to the nucleus. The more protons an atom has in its nucleus, the more strongly the electrons will be attracted and the more energy will be given out. As we know, atomic number is also known as proton number, and it is the amount of protons that determine the energy of the X-rays.
After years of searching, at last we had a periodic table that really worked, and the fact that we still use it today is testament to the huge achievement of these and many other great minds of the last two centuries of scientific discovery.